A novel orthotopic mouse model replicates human lung cancer cachexia
- PMID: 37025071
- PMCID: PMC10235890
- DOI: 10.1002/jcsm.13222
A novel orthotopic mouse model replicates human lung cancer cachexia
Abstract
Introduction: Cancer cachexia, highly prevalent in lung cancer, is a debilitating syndrome characterized by involuntary loss of skeletal muscle mass and is associated with poor clinical outcome, decreased survival and negative impact on tumour therapy. Various lung tumour-bearing animal models have been used to explore underlying mechanisms of cancer cachexia. However, these models do not simulate anatomical and immunological features key to lung cancer and associated muscle wasting. Overcoming these shortcomings is essential to translate experimental findings into the clinic. We therefore evaluated whether a syngeneic, orthotopic lung cancer mouse model replicates systemic and muscle-specific alterations associated with human lung cancer cachexia.
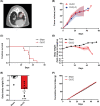
Methods: Immune competent, 11 weeks old male 129S2/Sv mice, were randomly allocated to either (1) sham control group or (2) tumour-bearing group. Syngeneic lung epithelium-derived adenocarcinoma cells (K-rasG12D ; p53R172HΔG ) were inoculated intrapulmonary into the left lung lobe of the mice. Body weight and food intake were measured daily. At baseline and weekly after surgery, grip strength was measured and tumour growth and muscle volume were assessed using micro cone beam CT imaging. After reaching predefined surrogate survival endpoint, animals were euthanized, and skeletal muscles of the lower hind limbs were collected for biochemical analysis.
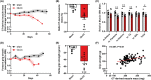
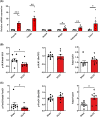
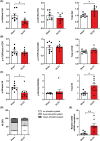
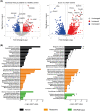
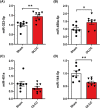
Results: Two-third of the tumour-bearing mice developed cachexia based on predefined criteria. Final body weight (-13.7 ± 5.7%; P < 0.01), muscle mass (-13.8 ± 8.1%; P < 0.01) and muscle strength (-25.5 ± 10.5%; P < 0.001) were reduced in cachectic mice compared with sham controls and median survival time post-surgery was 33.5 days until humane endpoint. Markers for proteolysis, both ubiquitin proteasome system (Fbxo32 and Trim63) and autophagy-lysosomal pathway (Gabarapl1 and Bnip3), were significantly upregulated, whereas markers for protein synthesis (relative phosphorylation of Akt, S6 and 4E-BP1) were significantly decreased in the skeletal muscle of cachectic mice compared with control. The cachectic mice exhibited increased pentraxin-2 (P < 0.001) and CXCL1/KC (P < 0.01) expression levels in blood plasma and increased mRNA expression of IκBα (P < 0.05) in skeletal muscle, indicative for the presence of systemic inflammation. Strikingly, RNA sequencing, pathway enrichment and miRNA expression analyses of mouse skeletal muscle strongly mirrored alterations observed in muscle biopsies of patients with lung cancer cachexia.
Conclusions: We developed an orthotopic model of lung cancer cachexia in immune competent mice. Because this model simulates key aspects specific to cachexia in lung cancer patients, it is highly suitable to further investigate the underlying mechanisms of lung cancer cachexia and to test the efficacy of novel intervention strategies.
Keywords: Cancer cachexia; Lung cancer; Mouse model; Muscle wasting; OLCC; Orthotopic mouse model.
© 2023 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
Wouter van de Worp, Jan Theys, Alba Sanz González, Brent van der Heyden, Florian Caiment, Duncan Hauser, Bert Smeets, Annemie Schols and Ramon Langen do not have any conflicts of interest. Frank Verhaegen is co‐founder of SmART Scientific Solutions B. V. Ardy van Helvoort is employed by Danone Nutricia Research.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

