Activation of mTORC1 Signaling Cascade in Hippocampus and Medial Prefrontal Cortex Is Required for Antidepressant Actions of Vortioxetine in Mice
- PMID: 37025079
- PMCID: PMC10586031
- DOI: 10.1093/ijnp/pyad017
Activation of mTORC1 Signaling Cascade in Hippocampus and Medial Prefrontal Cortex Is Required for Antidepressant Actions of Vortioxetine in Mice
Abstract
Background: Although thought of as a multimodal-acting antidepressant targeting the serotonin system, more molecules are being shown to participate in the antidepressant mechanism of vortioxetine. A previous report has shown that vortioxetine administration enhanced the expression of rapamycin complex 1 (mTORC1) in neurons. It has been well demonstrated that mTORC1 participates in not only the pathogenesis of depression but also the pharmacological mechanisms of many antidepressants. Therefore, we speculate that the antidepressant mechanism of vortioxetine may require mTORC1.
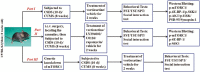
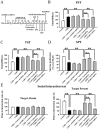
Methods: Two mouse models of depression (chronic social defeat stress and chronic unpredictable mild stress) and western blotting were first used together to examine whether vortioxetine administration produced reversal effects against the chronic stress-induced downregulation in the whole mTORC1 signaling cascade in both the hippocampus and medial prefrontal cortex (mPFC). Then, LY294002, U0126, and rapamycin were used together to explore whether the antidepressant effects of vortioxetine in mouse models of depression were attenuated by pharmacological blockade of the mTORC1 system. Furthermore, lentiviral-mTORC1-short hairpin RNA-enhanced green fluorescence protein (LV-mTORC1-shRNA-EGFP) was adopted to examine if genetic blockade of mTORC1 also abolished the antidepressant actions of vortioxetine in mice.
Results: Vortioxetine administration produced significant reversal effects against the chronic stress-induced downregulation in the whole mTORC1 signaling cascade in both the hippocampus and mPFC. Both pharmacological and genetic blockade of the mTORC1 system notably attenuated the antidepressant effects of vortioxetine in mice.
Conclusions: Activation of the mTORC1 system in the hippocampus and mPFC is required for the antidepressant actions of vortioxetine in mice.
Keywords: Chronic social defeat stress; chronic unpredictable mild stress; depression; mammalian target of rapamycin complex 1; vortioxetine.
© The Author(s) 2023. Published by Oxford University Press on behalf of CINP.
Figures







Similar articles
-
Venlafaxine protects against chronic stress-related behaviors in mice by activating the mTORC1 signaling cascade.J Affect Disord. 2020 Nov 1;276:525-536. doi: 10.1016/j.jad.2020.07.096. Epub 2020 Jul 19. J Affect Disord. 2020. PMID: 32871684
-
Intranasal Administration of Resolvin E1 Produces Antidepressant-Like Effects via BDNF/VEGF-mTORC1 Signaling in the Medial Prefrontal Cortex.Neurotherapeutics. 2023 Mar;20(2):484-501. doi: 10.1007/s13311-022-01337-1. Epub 2023 Jan 9. Neurotherapeutics. 2023. PMID: 36622634 Free PMC article.
-
Antidepressant-like effects of tomatidine and tomatine, steroidal alkaloids from unripe tomatoes, via activation of mTORC1 in the medial prefrontal cortex in lipopolysaccharide-induced depression model mice.Nutr Neurosci. 2024 Aug;27(8):795-808. doi: 10.1080/1028415X.2023.2254542. Epub 2023 Sep 13. Nutr Neurosci. 2024. PMID: 37704369
-
Role of mTOR1 signaling in the antidepressant effects of ketamine and the potential of mTORC1 activators as novel antidepressants.Neuropharmacology. 2023 Feb 1;223:109325. doi: 10.1016/j.neuropharm.2022.109325. Epub 2022 Nov 9. Neuropharmacology. 2023. PMID: 36334763 Review.
-
Regional distribution of serotonergic receptors: a systems neuroscience perspective on the downstream effects of the multimodal-acting antidepressant vortioxetine on excitatory and inhibitory neurotransmission.CNS Spectr. 2016 Apr;21(2):162-83. doi: 10.1017/S1092852915000486. Epub 2015 Aug 7. CNS Spectr. 2016. PMID: 26250622 Review.
References
-
- Abelaira HM, Réus GZ, Neotti MV, Quevedo J (2014) The role of mTOR in depression and antidepressant responses. Life Sci 101:10–14. - PubMed
-
- Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019) Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev 99:101–116. - PubMed
-
- Asati V, Mahapatra DK, Bharti SK (2016) PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: structural and pharmacological perspectives. Eur J Med Chem 109:314–341. - PubMed
-
- Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151. - PubMed
-
- Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, Collo G (2018) Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry 23:812–823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

