The fetal pain paradox
- PMID: 37025166
- PMCID: PMC10072285
- DOI: 10.3389/fpain.2023.1128530
The fetal pain paradox
Abstract
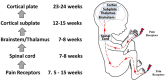
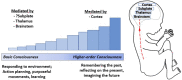
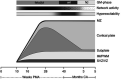
Controversy exists as to when conscious pain perception in the fetus may begin. According to the hypothesis of cortical necessity, thalamocortical connections, which do not form until after 24-28 weeks gestation, are necessary for conscious pain perception. However, anesthesiologists and neonatologists treat age-matched neonates as both conscious and pain-capable due to observable and measurable behavioral, hormonal, and physiologic indicators of pain. In preterm infants, these multimodal indicators of pain are uncontroversial, and their presence, despite occurring prior to functional thalamocortical connections, has guided the use of analgesics in neonatology and fetal surgery for decades. However, some medical groups state that below 24 weeks gestation, there is no pain capacity. Thus, a paradox exists in the disparate acknowledgment of pain capability in overlapping patient populations. Brain networks vary by age. During the first and second trimesters, the cortical subplate, a unique structure that is present only during fetal and early neonatal development, forms the first cortical network. In the third trimester, the cortical plate assumes this function. According to the subplate modulation hypothesis, a network of connections to the subplate and subcortical structures is sufficient to facilitate conscious pain perception in the fetus and the preterm neonate prior to 24 weeks gestation. Therefore, similar to other fetal and neonatal systems that have a transitional phase (i.e., circulatory system), there is now strong evidence for transitional developmental phases of fetal and neonatal pain circuitry.
Keywords: fetal analgesia; fetal anesthesia; fetal awareness; fetal nociception; fetal pain; subplate.
© 2023 Thill.
Conflict of interest statement
The author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Society for Maternal-Fetal Medicine (SMFM), Society of Family Planning (SFP), Norton ME, Cassidy A, Ralston SJ, Chatterjee D, et al. Society for maternal-fetal medicine consult series #59: the use of analgesia and anesthesia for maternal-fetal procedures. Am J Obstet Gynecol. (2021) 225(6):B2–B8. 10.1016/j.ajog.2021.08.031 - DOI - PubMed
-
- Royal College of Obstetricians and Gynaecologists. RCOG Fetal Awareness Evidence Review (2022). Available from: https://www.rcog.org.uk/guidance/browse-all-guidance/other-guidelines-an... (Accessed January 20, 2023).
-
- Bellieni CV. Foetal pain and anaesthesia during prenatal surgery. Clin Exp Obstet Gynecol. (2022) 49(4):79. 10.31083/j.ceog4904079 - DOI
Publication types
LinkOut - more resources
Full Text Sources

