Plasmonic nanotechnology for photothermal applications - an evaluation
- PMID: 37025366
- PMCID: PMC10071519
- DOI: 10.3762/bjnano.14.33
Plasmonic nanotechnology for photothermal applications - an evaluation
Abstract
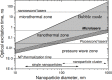
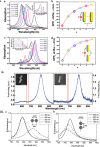
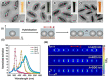
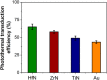
The application of plasmonic nanoparticles is motivated by the phenomenon of surface plasmon resonance. Owing to the tunability of optothermal properties and enhanced stability, these nanostructures show a wide range of applications in optical sensors, steam generation, water desalination, thermal energy storage, and biomedical applications such as photothermal (PT) therapy. The PT effect, that is, the conversion of absorbed light to heat by these particles, has led to thriving research regarding the utilization of plasmonic nanoparticles for a myriad of applications. The design of conventional nanomaterials for PT conversion has focussed predominantly on the manipulation of photon absorption through bandgap engineering, doping, incorporation, and modification of suitable matrix materials. Plasmonic nanomaterials offer an alternative and attractive approach in this regard, through the flexibility in the excitation of surface plasmons. Specific advantages are the considerable improved bandwidth of the absorption, a higher efficiency of photon absorption, facile tuning, as well as flexibility in the synthesis of plasmonic nanomaterials. This review of plasmonic PT (PPT) research begins with a theoretical discussion on the plasmonic properties of nanoparticles by means of the quasi-static approximation, Mie theory, Gans theory, generic simulations on common plasmonic material morphologies, and the evaluation processes of PT performance. Further, a variety of nanomaterials and material classes that have potential for PPT conversion are elucidated, such as plasmonic metals, bimetals, and metal-metal oxide nanocomposites. A detailed investigation of the essential, but often ignored, concept of thermal, chemical, and aggregation stability of nanoparticles is another part of this review. The challenges that remain, as well as prospective directions and chemistries, regarding nanomaterials for PT conversion are pondered on in the final section of the article, taking into account the specific requirements from different applications.
Keywords: nanoparticle heating; phonons; photothermal; plasmonic; stability; surface plasmon resonance.
Copyright © 2023, Indhu et al.
Figures


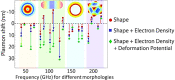
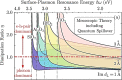


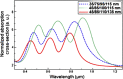


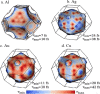














References
-
- Dirker J, Juggurnath D, Kaya A, Osowade E A, Simpson M, Lecompte S, Noori Rahim Abadi S M A, Voulgaropoulos V, Adelaja A O, Dauhoo M Z, et al. Front Energy Res. 2019;6:10.3389/fenrg.2018.00147. doi: 10.3389/fenrg.2018.00147. - DOI
-
- Gärtner W W. Phys Rev. 1961;122(2):419–424. doi: 10.1103/physrev.122.419. - DOI
-
- Cortezon-Tamarit F, Ge H, Mirabello V, Theobald M B M, Calatayud D G, Pascu S I. Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells. Academic Press; 2017. Carbon Nanotubes and Related Nanohybrids Incorporating Inorganic Transition Metal Compounds and Radioactive Species as Synthetic Scaffolds for Nanomedicine Design; pp. 245–327. - DOI
-
- Li S, Wang X, Hu R, Chen H, Li M, Wang J, Wang Y, Liu L, Lv F, Liang X-J, et al. Chem Mater. 2016;28(23):8669–8675. doi: 10.1021/acs.chemmater.6b03738. - DOI
Publication types
LinkOut - more resources
Full Text Sources
