Pathophysiology of Cav1.3 L-type calcium channels in the heart
- PMID: 37025382
- PMCID: PMC10070707
- DOI: 10.3389/fphys.2023.1144069
Pathophysiology of Cav1.3 L-type calcium channels in the heart
Abstract
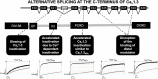
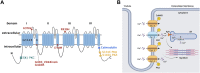
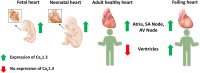
Ca2+ plays a crucial role in excitation-contraction coupling in cardiac myocytes. Dysfunctional Ca2+ regulation alters the force of contraction and causes cardiac arrhythmias. Ca2+ entry into cardiomyocytes is mediated mainly through L-type Ca2+ channels, leading to the subsequent Ca2+ release from the sarcoplasmic reticulum. L-type Ca2+ channels are composed of the conventional Cav1.2, ubiquitously expressed in all heart chambers, and the developmentally regulated Cav1.3, exclusively expressed in the atria, sinoatrial node, and atrioventricular node in the adult heart. As such, Cav1.3 is implicated in the pathogenesis of sinoatrial and atrioventricular node dysfunction as well as atrial fibrillation. More recently, Cav1.3 de novo expression was suggested in heart failure. Here, we review the functional role, expression levels, and regulation of Cav1.3 in the heart, including in the context of cardiac diseases. We believe that the elucidation of the functional and molecular pathways regulating Cav1.3 in the heart will assist in developing novel targeted therapeutic interventions for the aforementioned arrhythmias.
Keywords: atrial fibrillation; calcium channel; heart failure; protein kinase regulation; sinoatrial node dysfunction.
Copyright © 2023 Zaveri, Srivastava, Qu, Chahine and Boutjdir.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

