Current concepts in the pathogenesis of periodontitis: from symbiosis to dysbiosis
- PMID: 37025387
- PMCID: PMC10071981
- DOI: 10.1080/20002297.2023.2197779
Current concepts in the pathogenesis of periodontitis: from symbiosis to dysbiosis
Abstract
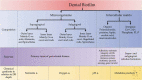
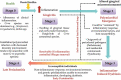
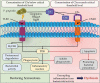
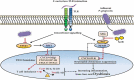
The primary etiological agent for the initiation and progression of periodontal disease is the dental plaque biofilm which is an organized aggregation of microorganisms residing within a complex intercellular matrix. The non-specific plaque hypothesis was the first attempt to explain the role of the dental biofilm in the pathogenesis of periodontal diseases. However, the introduction of sophisticated diagnostic and laboratory assays has led to the realisation that the development of periodontitis requires more than a mere increase in the biomass of dental plaque. Indeed, multispecies biofilms exhibit complex interactions between the bacteria and the host. In addition, not all resident microorganisms within the biofilm are pathogenic, since beneficial bacteria exist that serve to maintain a symbiotic relationship between the plaque microbiome and the host's immune-inflammatory response, preventing the emergence of pathogenic microorganisms and the development of dysbiosis. This review aims to highlight the development and structure of the dental plaque biofilm and to explore current literature on the transition from a healthy (symbiotic) to a diseased (dysbiotic) biofilm in periodontitis and the associated immune-inflammatory responses that drive periodontal tissue destruction and form mechanistic pathways that impact other systemic non-communicable diseases.
Keywords: Dental biofilm; dysbiosis; inflammation; periodontal disease; symbiosis.
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol. 2013;84(4 Suppl):S106–112. - PubMed
-
- Hirschfeld J, Chapple IL. Periodontitis and systemic diseases: clinical evidence and biological plausibility. Berlin: Quintessenz Verlag; 2021.
Publication types
LinkOut - more resources
Full Text Sources
Medical
