Effects of combined binding of chlorogenic acid/caffeic acid and gallic acid to trypsin on their synergistic antioxidant activity, enzyme activity and stability
- PMID: 37025419
- PMCID: PMC10070516
- DOI: 10.1016/j.fochx.2023.100664
Effects of combined binding of chlorogenic acid/caffeic acid and gallic acid to trypsin on their synergistic antioxidant activity, enzyme activity and stability
Abstract
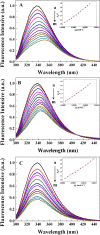
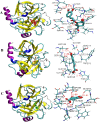
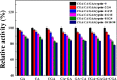
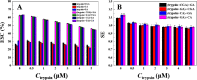
The combined application of multiple natural polyphenols in functional foods may provide better health benefits. The binding of polyphenols with different structures to proteins will affect their respective functions. Spectroscopy and molecular docking were used to investigate the competitive binding of chlorogenic acid (CGA)/caffeic acid (CA) and gallic acid (GA) to trypsin. The effects of different molecular structures and the order of adding the three phenolic acids on the binding were assessed. The stability of trypsin and its docked complexes with CGA/CA/GA was evaluated by molecular dynamics simulation. The effects of the binding process on the activity and thermal stability of trypsin, as well as on the antioxidant activity and stability of CGA/CA/GA were explored. The competitive binding of CGA/CA and GA to trypsin affected their synergistic antioxidant effects. The results may provide a reference for the combined application of CGA/CA and GA in food and pharmaceutical fields.
Keywords: Activity; Caffeic acid (CA, PubChem CID: 689043); Chlorogenic acid (CGA, PubChem CID: 1794427); Combined binding; Gallic acid (GA, PubChem CID: 370); Phenolic acids; Stability; Trypsin.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Complexation of bovine lactoferrin with selected phenolic acids via noncovalent interactions: Binding mechanism and altered functionality.J Dairy Sci. 2024 Jul;107(7):4189-4204. doi: 10.3168/jds.2023-24088. Epub 2024 Feb 17. J Dairy Sci. 2024. PMID: 38369115
-
Covalent modification of zein with polyphenols: A feasible strategy to improve antioxidant activity and solubility.J Food Sci. 2022 Jul;87(7):2965-2979. doi: 10.1111/1750-3841.16203. Epub 2022 May 31. J Food Sci. 2022. PMID: 35638335
-
Understanding structures and thermodynamics of β-cyclodextrin encapsulation of chlorogenic, caffeic and quinic acids: Implications for enriching antioxidant capacity and masking bitterness in coffee.Food Chem. 2019 Sep 30;293:550-560. doi: 10.1016/j.foodchem.2019.04.084. Epub 2019 Apr 24. Food Chem. 2019. PMID: 31151647
-
Polyphenols and their applications: An approach in food chemistry and innovation potential.Food Chem. 2021 Feb 15;338:127535. doi: 10.1016/j.foodchem.2020.127535. Epub 2020 Jul 9. Food Chem. 2021. PMID: 32798817 Review.
-
Functionalization of sweet potato leaf polyphenols by nanostructured composite β-lactoglobulin particles from molecular level complexations: A review.Food Chem. 2022 Mar 15;372:131304. doi: 10.1016/j.foodchem.2021.131304. Epub 2021 Oct 4. Food Chem. 2022. PMID: 34655825 Review.
Cited by
-
Combined Experimental and Theoretical Insights: Spectroscopic and Molecular Investigation of Polyphenols from Fagonia indica via DFT, UV-vis, and FT-IR Approaches.ACS Omega. 2023 Dec 29;9(1):730-740. doi: 10.1021/acsomega.3c06544. eCollection 2024 Jan 9. ACS Omega. 2023. PMID: 38222607 Free PMC article.
-
The hemostatic molecular mechanism of Sanguisorbae Radix's pharmacological active components based on HSA: Spectroscopic investigations, molecular docking and dynamics simulation.Heliyon. 2024 Aug 28;10(17):e37020. doi: 10.1016/j.heliyon.2024.e37020. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296229 Free PMC article.
-
Phytochemical profiling and fractionation of Helianthemum lippii extract versus silver nanoparticle-modified extract: assessment of photoprotective, anti-hemolytic, antibacterial, and anti-inflammatory properties.Front Chem. 2024 Dec 10;12:1508707. doi: 10.3389/fchem.2024.1508707. eCollection 2024. Front Chem. 2024. PMID: 39720555 Free PMC article.
-
Bioactive Properties of Persea americana Peel Extract and Their Role in Hypercholesterolemia Management and Cardiovascular Health.Foods. 2025 Jul 16;14(14):2482. doi: 10.3390/foods14142482. Foods. 2025. PMID: 40724303 Free PMC article.
-
Investigation of the Impact of Saccharides on the Relative Activity of Trypsin and Catalase after Droplet and Spray Drying.Pharmaceutics. 2023 Oct 21;15(10):2504. doi: 10.3390/pharmaceutics15102504. Pharmaceutics. 2023. PMID: 37896264 Free PMC article.
References
-
- Ahmat A.M., Thiebault T., Guégan R. Phenolic acids interactions with clay minerals: A spotlight on the adsorption mechanisms of Gallic Acid onto montmorillonite. Applied Clay Science. 2019;180 doi: 10.1016/j.clay.2019.105188. - DOI
-
- Ameen F., Siddiqui S., Jahan I., Nayeem S.M., Rehman S., Tabish M. A detailed insight into the interaction of memantine with bovine serum albumin: A spectroscopic and computational approach. Journal of Molecular Liquids. 2020;303 doi: 10.1016/j.molliq.2020.112671. - DOI
-
- Chen J., Chen Y., Li S.Y., Yang J., Dong J.B. In-situ growth of cerium-based metal organic framework on multi-walled carbon nanotubes for electrochemical detection of gallic acid. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2022;650 doi: 10.1016/j.colsurfa.2022.129318. - DOI
-
- Goswami S., Ghoshb A., Boraha K., Mahantaa A., Guhac A.K., Bora S.J. Structure-property correlation in gallic acid and 4-cyanopyridine cocrystal and binding studies with drug efflux pump in bacteria. Journal of Molecular Structure. 2021;1225 doi: 10.1016/j.molstruc.2020.129279. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

