Wearable cardioverter defibrillator for preventing sudden cardiac death in patients at risk: An updated systematic review of comparative effectiveness and safety
- PMID: 37025482
- PMCID: PMC10070821
- DOI: 10.1016/j.ijcha.2023.101189
Wearable cardioverter defibrillator for preventing sudden cardiac death in patients at risk: An updated systematic review of comparative effectiveness and safety
Abstract
Objectives: To synthesise the available evidence of wearable cardioverter defibrillator (WCD) therapy as an add-on measure to optimal medical therapy (OMT) or as a replacement of hospital stay.
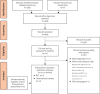
Methods: An update systematic review (SR) of comparative effectiveness and safety of WCD therapy was conducted. We included randomised controlled trials (RCT), prospective comparative studies and prospective uncontrolled studies with at least 100 patients. A narrative synthesis of the evidence was conducted.
Results: One RCT (n = 2348) and further eleven observational studies (n = 5345) fulfilled our inclusion criteria. In the only available RCT, the use of the WCD was not statistically associated with a clinical benefit on arrhythmic mortality in post-myocardial infarction (MI) patients with an ejection fraction of ≤35%. The compliance with WCD therapy was low in the RCT and high in observational studies, with ten observational studies reporting on a daily wear time between 20 and 23.5 h. The range of percentage of patients receiving at least one appropriate shock was 1-4.8% and the rate of first shock success was reported to be 100% in three studies. Serious adverse events (SAEs) such as inappropriate shocks occurred rarely, with between 0% and 2% of patients being inappropriately shocked within ten observational studies. In one of the observational studies, two patients (2%) were allergic to nickel developing skin rash and false alarms occurred in 58 patients (57%) in this study. Another registry study (n = 448) reported milder AEs, such as dermatitis and pressure marks, occurring in 0.9% and 0.2% of enrolled patients, respectively.
Conclusion: The only available RCT failed to show superiority of add-on use of WCD in post MI patients. Observational evidence shows that the compliance with WCD is good, but the evidence is afflicted with selection bias and the inclusion of diverse mixed patient populations diluting the ability to draw indication-specific conclusions on the utility of the device. More comparative data is needed to justify continuing or expanding use of WCD therapy.
Keywords: (cardioverter-) defibrillator (external; Countershock; Sudden cardiac arrest; Ventricular fibrillation; Ventricular tachycardia; Wearable).
© 2023 HTA Austria - Austrian Institute for Health Technology Assessment GmbH.
Conflict of interest statement
None declared.
Figures



References
-
- Priori S.G., Blomström-Lundqvist C. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur. Heart J. 2015;36(41):2757–2759. doi: 10.1093/eurheartj/ehv445. Epub 2016/01/09. - DOI - PubMed
-
- ZOLL Medical Corporation LifeVest Model 4000 - Patient Manual. 2023. https://lifevest.zoll.com/patients [cited 01.08.2022]. Available from:
-
- Chiarolla E., Orso M., Götz G., Stanak M., Wild C., Jefferson T. Decision Support Document 103/ Update 2018. 2019. Wearable cardioverter-defibrillator (WCD) therapy in primary and secondary prevention of sudden cardiac arrest in patients at risk. Update 2018.https://eprints.aihta.at/1186/ [cited 15.04.2022]. Available from:
-
- Food and Drug Administration (FDA) ASSURE Wearable Cardioverter Defibrillator System Kit. 2021. https://www.fda.gov/medical-devices/recently-approved-devices/assure-wea... Available from:
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

