Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status
- PMID: 37025630
- PMCID: PMC10072159
- DOI: 10.3389/fmicb.2023.1135693
Cold-tolerant phosphate-solubilizing Pseudomonas strains promote wheat growth and yield by improving soil phosphorous (P) nutrition status
Abstract
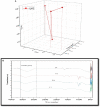
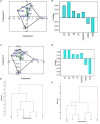
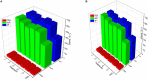
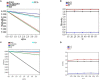
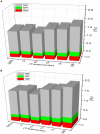
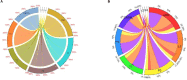
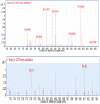
It is well-known that phosphate-solubilizing bacteria (PSB) promote crop growth and yield. The information regarding characterization of PSB isolated from agroforestry systems and their impact on wheat crops under field conditions is rarely known. In the present study, we aim to develop psychrotroph-based P biofertilizers, and for that, four PSB strains (Pseudomonas sp. L3, Pseudomonas sp. P2, Streptomyces sp. T3, and Streptococcus sp. T4) previously isolated from three different agroforestry zones and already screened for wheat growth under pot trial conditions were evaluated on wheat crop under field conditions. Two field experiments were employed; set 1 includes PSB + recommended dose of fertilizers (RDF) and set 2 includes PSB - RDF. In both field experiments, the response of the PSB-treated wheat crop was significantly higher compared to the uninoculated control. In field set 1, an increase of 22% in grain yield (GY), 16% in biological yield (BY), and 10% in grain per spike (GPS) was observed in consortia (CNS, L3 + P2) treatment, followed by L3 and P2 treatments. Inoculation of PSB mitigates soil P deficiency as it positively influences soil alkaline phosphatase (AP) and soil acid phosphatase (AcP) activity which positively correlated with grain NPK %. The highest grain NPK % was reported in CNS-treated wheat with RDF (N-0.26%, P-0.18%, and K-1.66%) and without RDF (N-0.27, P-0.26, and K-1.46%), respectively. All parameters, including soil enzyme activities, plant agronomic data, and yield data were analyzed by principal component analysis (PCA), resulting in the selection of two PSB strains. The conditions for optimal P solubilization, in L3 (temperature-18.46, pH-5.2, and glucose concentration-0.8%) and P2 (temperature-17°C, pH-5.0, and glucose concentration-0.89%), were obtained through response surface methodology (RSM) modeling. The P solubilizing potential of selected strains at <20°C makes them a suitable candidate for the development of psychrotroph-based P biofertilizers. Low-temperature P solubilization of the PSB strains from agroforestry systems makes them potential biofertilizers for winter crops.
Keywords: PSB; fertilizer; principal component analysis; psychrotroph; response surface methodology.
Copyright © 2023 Dasila, Sah, Jaggi, Kumar, Tewari, Taj, Chaturvedi, Perveen, Bukhari, Siang and Sahgal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Agri U., Chaudhary P., Sharma A., Kukreti B. (2022). Physiological response of maize plants and its rhizospheric microbiome under the influence of potential bioinoculants and nanochitosan. Plant Soil 474, 451–468. 10.1007/s11104-022-05351-2 - DOI
-
- Akhtar M. S., Oki Y., Nakashima Y., Adachi T., Nishigaki M. (2016). Phosphorus stress-induced differential growth, and phosphorus acquisition and use efficiency by spring wheat cultivars. Commun. Soil Sci. Plant Anal. 47, 15–27. 10.1080/00103624.2016.1232089 - DOI
-
- Akintokun A. K., Akande G. A., Akintokun P. O., Popoola T. O. S., Babalola A. O. (2007). Solubilization of insoluble phosphate by organic acid-producing fungi isolated from Nigerian soil. Int. J. Soil Sci. 2, 301–307. 10.3923/ijss.2007.301.307 - DOI
LinkOut - more resources
Full Text Sources

