Ring forming transformations of ynamides via cycloaddition
- PMID: 37025669
- PMCID: PMC10072253
- DOI: 10.1039/d3ra00139c
Ring forming transformations of ynamides via cycloaddition
Abstract
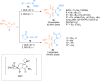
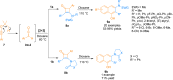
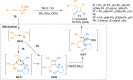
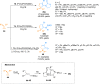
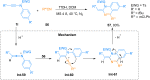
Ynamides are N-alkyne compounds bearing an electron withdrawing group at the nitrogen atom. They offer unique pathways for the construction of versatile building blocks owing to their exceptional balance between reactivity and stability. Recently several studies have been reported that explore and illustrate the synthetic potential of ynamides and ynamide-derived advanced intermediates in cycloadditions with different reaction partners to yield heterocyclic cycloadducts of synthetic and pharmaceutical value. Cycloaddition reactions of ynamides are the facile and preferable routes for the construction of structural motifs having striking importance in synthetic, medicinal chemistry, and advanced materials. In this systematic review, we highlighted the recently reported novel transformations and synthetic applications that involved the cycloaddition reaction of ynamides. The scope along with the limitations of the transformations are discussed in detail.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



















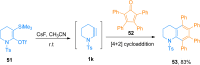






















Similar articles
-
1,3-Butadiynamides the Ethynylogous Ynamides: Synthesis, Properties and Applications in Heterocyclic Chemistry.Molecules. 2023 Jun 5;28(11):4564. doi: 10.3390/molecules28114564. Molecules. 2023. PMID: 37299038 Free PMC article. Review.
-
Ynamides in ring forming transformations.Acc Chem Res. 2014 Feb 18;47(2):560-78. doi: 10.1021/ar400193g. Epub 2013 Oct 28. Acc Chem Res. 2014. PMID: 24164363 Free PMC article.
-
Ynamides as Three-Atom Components in Cycloadditions: An Unexplored Chemical Reaction Space.J Am Chem Soc. 2021 Jun 16. doi: 10.1021/jacs.1c04051. Online ahead of print. J Am Chem Soc. 2021. PMID: 34132536
-
S3S63 Terminal Ynamides: Synthesis, Coupling Reactions and Additions to Common Electrophiles.Tetrahedron Lett. 2015 May 6;56(19):2377-2392. doi: 10.1016/j.tetlet.2015.03.111. Tetrahedron Lett. 2015. PMID: 26085692 Free PMC article.
-
Reactivity of ynamides in catalytic intermolecular annulations.Chem Soc Rev. 2021 Mar 1;50(4):2582-2625. doi: 10.1039/d0cs00283f. Chem Soc Rev. 2021. PMID: 33367365 Review.
Cited by
-
1,3-Butadiynamides the Ethynylogous Ynamides: Synthesis, Properties and Applications in Heterocyclic Chemistry.Molecules. 2023 Jun 5;28(11):4564. doi: 10.3390/molecules28114564. Molecules. 2023. PMID: 37299038 Free PMC article. Review.
-
Pallado-Catalyzed Cascade Synthesis of 2-Alkoxyquinolines from 1,3-Butadiynamides.Molecules. 2024 Jul 26;29(15):3505. doi: 10.3390/molecules29153505. Molecules. 2024. PMID: 39124910 Free PMC article.
References
-
- Evano G. Jouvin K. Coste A. Synthesis. 2012;45:17–26.
-
- Hsung R. Mulder J. Kurtz K. Synlett. 2003:1379–1390.
-
- Evano G. Blanchard N. Toumi M. Chem. Rev. 2008;108:3054–3131. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

