PCSK9 regulates the efficacy of immune checkpoint therapy in lung cancer
- PMID: 37025995
- PMCID: PMC10070680
- DOI: 10.3389/fimmu.2023.1142428
PCSK9 regulates the efficacy of immune checkpoint therapy in lung cancer
Abstract
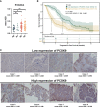
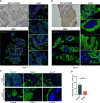
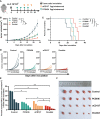
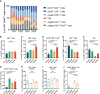
Proprotein convertase subtilisin/kexin type 9 (PCSK9) secreted by tumors was reported as a deleterious factor that led to the reduction of lymphocyte infiltration and the poorer efficacy of ICIs in vivo. This study aimed to explore whether PCSK9 expression in tumor tissue could predict the response of advanced non-small cell lung cancer (NSCLC) to anti-PD-1 immunotherapy and the synergistic antitumor effect of the combination of the PCSK9 inhibitor with the anti-CD137 agonist. One hundred fifteen advanced NSCLC patients who received anti-PD-1 immunotherapy were retrospectively studied with PCSK9 expression in baseline NSCLC tissues detected by immunohistochemistry (IHC). The mPFS of the PCSK9lo group was significantly longer than that of the PCSK9hi group [8.1 vs. 3.6 months, hazard ratio (HR): 3.450; 95% confidence interval (CI), 2.166-5.496]. A higher objective response rate (ORR) and a higher disease control rate (DCR) were observed in the PCSK9lo group than in the PCSK9hi group (54.4% vs. 34.5%, 94.7% vs. 65.5%). Reduction and marginal distribution of CD8+ T cells were observed in PCSK9hi NSCLC tissues. Tumor growth was retarded by the PCSK9 inhibitor and the anti-CD137 agonist alone in the Lewis lung carcinoma (LLC) mice model and further retarded by the PCSK9 inhibitor in combination with the CD137 agonist with long-term survival of the host mice with noticeable increases of CD8+ and GzmB+ CD8+ T cells and reduction of Tregs. Together, these results suggested that high PCSK9 expression in baseline tumor tissue was a deleterious factor for the efficacy of anti-PD-1 immunotherapy in advanced NSCLC patients. The PCSK9 inhibitor in combination with the anti-CD137 agonist could not only enhance the recruitment of CD8+ and GzmB+ CD8+ T cells but also deplete Tregs, which may be a novel therapeutic strategy for future research and clinical practice.
Keywords: PCSK9; advanced non-small cell lung cancer; anti-CD137 agonist; immune infiltration; immunohistochemical markers; immunotherapy; proprotein convertase subtilisin/kexin type 9.
Copyright © 2023 Gao, Yi, Jiang, Li, Wang, Yang, Li, Che, Wang, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Is Associated With Recurrence and Survival of Resectable Non-Small Cell Lung Cancer (NSCLC): A Retrospective Study.J Surg Res. 2024 Sep;301:231-239. doi: 10.1016/j.jss.2024.06.005. Epub 2024 Jul 4. J Surg Res. 2024. PMID: 38968924
-
Low baseline plasma PCSK9 level is associated with good clinical outcomes of immune checkpoint inhibitors in advanced non-small cell lung cancer.Thorac Cancer. 2022 Feb;13(3):353-360. doi: 10.1111/1759-7714.14259. Epub 2021 Dec 27. Thorac Cancer. 2022. PMID: 34962050 Free PMC article.
-
Inhibition of PCSK9 enhances the antitumor effect of PD-1 inhibitor in colorectal cancer by promoting the infiltration of CD8+ T cells and the exclusion of Treg cells.Front Immunol. 2022 Aug 8;13:947756. doi: 10.3389/fimmu.2022.947756. eCollection 2022. Front Immunol. 2022. PMID: 36003387 Free PMC article.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
Cited by
-
A Critical Review of Immunomodulation in the Management of Inoperable Stage III NSCLC.Cancers (Basel). 2025 May 30;17(11):1829. doi: 10.3390/cancers17111829. Cancers (Basel). 2025. PMID: 40507315 Free PMC article. Review.
-
The multifaceted role of PCSK9 in cancer pathogenesis, tumor immunity, and immunotherapy.Med Oncol. 2024 Jul 15;41(8):202. doi: 10.1007/s12032-024-02435-0. Med Oncol. 2024. PMID: 39008137 Review.
-
Neoadjuvant, Perioperative, and Adjuvant Immunotherapy in Early-Stage Surgically Resectable Non-Small Cell Lung Cancer: Updates and Future Perspectives.Cancers (Basel). 2025 Jun 21;17(13):2077. doi: 10.3390/cancers17132077. Cancers (Basel). 2025. PMID: 40647378 Free PMC article. Review.
-
Association of lipid-lowering drug targets with risk of cutaneous melanoma: a mendelian randomization study.BMC Cancer. 2024 May 17;24(1):602. doi: 10.1186/s12885-024-12366-8. BMC Cancer. 2024. PMID: 38760735 Free PMC article.
-
Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): from bench to bedside.Signal Transduct Target Ther. 2024 Jan 8;9(1):13. doi: 10.1038/s41392-023-01690-3. Signal Transduct Target Ther. 2024. PMID: 38185721 Free PMC article. Review.
References
-
- Abifadel M, Guerin M, Benjannet S, Rabes JP, Le Goff W, Julia Z, et al. . Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia. Atherosclerosis (2012) 223:394–400. doi: 10.1016/j.atherosclerosis.2012.04.006 - DOI - PubMed
-
- Xu B, Li S, Fang Y, Zou Y, Song D, Zhang S, et al. . Proprotein convertase Subtilisin/Kexin type 9 promotes gastric cancer metastasis and suppresses apoptosis by facilitating MAPK signaling pathway through HSP70 up-regulation. Front Oncol (2020) 10:609663. doi: 10.3389/fonc.2020.609663 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

