Ion channels in dry eye disease
- PMID: 37026252
- PMCID: PMC10276728
- DOI: 10.4103/IJO.IJO_3020_22
Ion channels in dry eye disease
Abstract
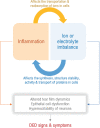
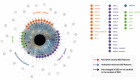
Dry eye disease (DED) which affects millions of people worldwide is an ocular surface disease that is strongly associated with pain, discomfort, and visual disturbances. Altered tear film dynamics, hyperosmolarity, ocular surface inflammation, and neurosensory abnormalities are the key contributors to DED pathogenesis. The presence of discordance between signs and symptoms of DED in patients and refractoriness to current therapies in some patients underpin the need for studying additional contributors that can be modulated. The presence of electrolytes or ions including sodium, potassium, chloride, bicarbonate, calcium, and magnesium in the tear fluid and ocular surface cells contribute to ocular surface homeostasis. Ionic or electrolyte imbalance and osmotic imbalance have been observed in DED and feed-forward interaction between ionic imbalances and inflammation alter cellular processes in the ocular surface resulting in DED. Ionic balances in various cellular and intercellular compartments are maintained by dynamic transport via ion channel proteins present in cell membranes. Hence, alterations in the expression and/or activity of about 33 types of ion channels that belong to voltage-gated channels, ligand-gated channels, mechanosensitive ion channel, aquaporins, chloride ion channel, sodium-potassium-chloride pumps or cotransporters have been investigated in the context of ocular surface health and DED in animal and/or human subjects. An increase in the expression or activity of TRPA1, TRPV1, Nav1.8, KCNJ6, ASIC1, ASIC3, P2X, P2Y, and NMDA receptor have been implicated in DED pathogenesis, whereas an increase in the expression or activity of TRPM8, GABAA receptor, CFTR, and NKA have been associated with resolution of DED.
Keywords: Dry eye disease; electrolytes; ion channels; ions; transient receptor potential.
Conflict of interest statement
None
Figures
References
-
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334–65. - PubMed
-
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83. - PubMed
-
- Clayton JA. Dry eye. N Engl J Med. 2018;378:2212–23. - PubMed
-
- Galor A. Painful dry eye symptoms:A nerve problem or a tear problem? Ophthalmology. 2019;126:648–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

