Effect of sample volume and time on rumen juice analysis in cattle
- PMID: 37026411
- PMCID: PMC10229352
- DOI: 10.1111/jvim.16697
Effect of sample volume and time on rumen juice analysis in cattle
Abstract
Background: Rumen juice analysis (RJA) involves analysis of a 10mL sample within minutes after sampling. However, it can be challenging to collect 10 mL of rumen juice (RJ) from some ruminants, and clinical circumstances can delay RJA.
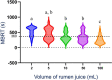
Objectives: Quantify the effect of sample volume (2, 5, 10, 50, and 100 mL), and time-to-analysis (0, 30, and 60 minutes) on RJA.
Animals: Cannulated cow.
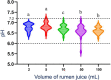
Methods: Observational experimental study. Two liters of RJ were collected at 26 separate times. The samples were subdivided into 2 duplicates of each sample volume at each sampling time; and analyzed at 0, 30, and 60 minutes after collection. Rumen juice analysis included pH measurement, methylene blue reduction time (MBRT), and protozoal motility.
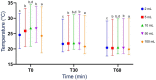
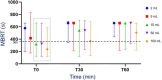
Results: The pH of 2 and 5 mL samples was significantly (P = .01) higher than the pH of 50 and 100 mL samples at all time points. The MBRT was significantly lower (faster bacterial reduction) for 100 mL samples compared to all other samples at 0 minute and to 2, 5, and 50 mL samples at 30 min. The pH and MBRT at 60 minutes were significantly higher than at 0 minute for all volumes (P < .05 and P < .01, respectively). For large protozoa, small sample volumes (2 and 5 mL) had significantly lower protozoal motility (scores of 5 and 4.5, respectively) compared to 100 mL samples at 60 minutes (score of 4; P < .05).
Conclusions and clinical importance: Interpretation of RJA could be affected by small sample volumes and delays to analysis. Sample volumes of ≥10 mL analyzed within 30 minutes after collection are recommended.
Keywords: bovine; forestomach indigestion; protozoa motility; rumen fluid; rumen sampling.
© 2023 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Munashe Chigerwe serves as Associate Editor for the Journal of Veterinary Internal Medicine. He was not involved in review of this manuscript. No other authors declare a conflict of interest.
Authors declare no conflict of interest.
Figures


Similar articles
-
Bacterial protein degradation by different rumen protozoal groups.J Anim Sci. 2012 Dec;90(12):4495-504. doi: 10.2527/jas.2012-5118. Epub 2012 Jul 24. J Anim Sci. 2012. PMID: 22829613
-
Negative correlation between protozoal and bacterial levels in rumen samples and its relation to the determination of dietary effects on the rumen microbial population.Appl Environ Microbiol. 1984 Mar;47(3):566-70. doi: 10.1128/aem.47.3.566-570.1984. Appl Environ Microbiol. 1984. PMID: 6712223 Free PMC article.
-
Assessment of ruminal bacterial populations and protozoal generation time in cows fed different methionine sources.J Dairy Sci. 2007 Feb;90(2):798-809. doi: 10.3168/jds.S0022-0302(07)71564-3. J Dairy Sci. 2007. PMID: 17235157
-
Impact of high-concentrate feeding and low ruminal pH on methanogens and protozoa in the rumen of dairy cows.Microb Ecol. 2011 Jul;62(1):94-105. doi: 10.1007/s00248-011-9881-0. Epub 2011 May 31. Microb Ecol. 2011. PMID: 21625972
-
Amino acid pattern of rumen microorganisms in cattle fed mixed diets-An update.J Anim Physiol Anim Nutr (Berl). 2022 Jul;106(4):752-771. doi: 10.1111/jpn.13676. Epub 2021 Dec 28. J Anim Physiol Anim Nutr (Berl). 2022. PMID: 34964170 Review.
Cited by
-
Impact of ginger powder (Zingiber officinale) supplementation on the performance, biochemical parameters, antioxidant status, and rumen fermentation in Ossimi rams.Vet World. 2024 Jul;17(7):1619-1628. doi: 10.14202/vetworld.2024.1619-1628. Epub 2024 Jul 26. Vet World. 2024. PMID: 39185052 Free PMC article.

