NCoA3 upregulation in breast cancer‑associated adipocytes elicits an inflammatory profile
- PMID: 37026525
- PMCID: PMC10091077
- DOI: 10.3892/or.2023.8542
NCoA3 upregulation in breast cancer‑associated adipocytes elicits an inflammatory profile
Abstract
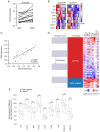
Nuclear receptor coactivator 3 (NCoA3) is a transcriptional coactivator of NF‑κB and other factors, which is expressed at relatively low levels in normal cells and is amplified or overexpressed in several types of cancer, including breast tumors. NCoA3 levels have been shown to be decreased during adipogenesis; however, its role in tumor‑surrounding adipose tissue (AT) remains unknown. Therefore, the present study assessed the modulation of NCoA3 in breast cancer‑associated adipocytes and evaluated its association with the expression of inflammatory markers. 3T3‑L1 adipocytes were stimulated with conditioned medium from human breast cancer cell lines and the expression levels of NCoA3 were evaluated by reverse transcription‑quantitative (q)PCR. NF‑κB activation was measured by immunofluorescence, and tumor necrosis factor and monocyte chemoattractant protein 1 levels were analyzed by qPCR and dot blot assays. The results obtained from the in vitro model were supported using mammary AT (MAT) from female mice, MAT adjacent to tumors from patients with breast cancer and bioinformatics analysis. The results revealed that adipocytes expressing high levels of NCoA3 were mainly associated with a pro‑inflammatory profile. In 3T3‑L1 adipocytes, NCoA3 downregulation or NF‑κB inhibition reversed the expression of inflammatory molecules. In addition, MAT from patients with a worse prognosis exhibited high levels of this coactivator. Notably, adipocyte NCoA3 levels could be modulated by inflammatory signals from tumors. The modulation of NCoA3 levels in synergy with NF‑κB activity in MAT in a tumor context could be factors required to establish breast cancer‑associated inflammation. As adipocytes are involved in the development and progression of breast cancer, this signaling network deserves to be further investigated to improve future tumor treatments.
Keywords: NF‑κB; breast cancer; inflammation; mammary adipose tissue; nuclear receptor coactivator 3.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Berstein LM, Kovalevskij AY, Poroshina TE, Kotov AV, Kovalenko IG, Tsyrlina EV, Leenman EE, Revskoy SY, Semiglazov VF, Pozharisski KM. Signs of proinflammatory/genotoxic switch (adipogenotoxicosis) in mammary fat of breast cancer patients: Role of menopausal status, estrogens and hyperglycemia. Int J Cancer. 2007;121:514–519. doi: 10.1002/ijc.22552. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

