Skeletal muscle adaptation to indirect electrical stimulation: divergence between microvascular and metabolic adaptations
- PMID: 37026596
- PMCID: PMC10988499
- DOI: 10.1113/EP091134
Skeletal muscle adaptation to indirect electrical stimulation: divergence between microvascular and metabolic adaptations
Abstract
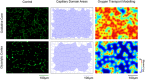
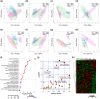
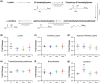
New findings: What is the central question of this study? Can we manipulate muscle recruitment to differentially enhance skeletal muscle fatigue resistance? What is the main finding and its importance? Through manipulation of muscle activation patterns, it is possible to promote distinct microvascular growth. Enhancement of fatigue resistance is closely associated with the distribution of the capillaries within the muscle, not necessarily with quantity. Additionally, at the acute stages of remodelling in response to indirect electrical stimulation, the improvement in fatigue resistance appears to be primarily driven by vascular remodelling, with metabolic adaptation of secondary importance.
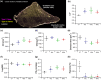
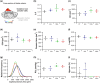
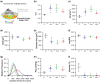
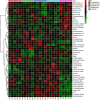
Abstract: Exercise involves a complex interaction of factors influencing muscle performance, where variations in recruitment pattern (e.g., endurance vs. resistance training) may differentially modulate the local tissue environment (i.e., oxygenation, blood flow, fuel utilization). These exercise stimuli are potent drivers of vascular and metabolic change. However, their relative contribution to adaptive remodelling of skeletal muscle and subsequent performance is unclear. Using implantable devices, indirect electrical stimulation (ES) of locomotor muscles of rat at different pacing frequencies (4, 10 and 40 Hz) was used to differentially recruit hindlimb blood flow and modulate fuel utilization. After 7 days, ES promoted significant remodelling of microvascular composition, increasing capillary density in the cortex of the tibialis anterior by 73%, 110% and 55% for the 4 Hz, 10 and 40 Hz groups, respectively. Additionally, there was remodelling of the whole muscle metabolome, including significantly elevated amino acid turnover, with muscle kynurenic acid levels doubled by pacing at 10 Hz (P < 0.05). Interestingly, the fatigue index of skeletal muscle was only significantly elevated in 10 Hz (58% increase) and 40 Hz (73% increase) ES groups, apparently linked to improved capillary distribution. These data demonstrate that manipulation of muscle recruitment pattern may be used to differentially expand the capillary network prior to altering the metabolome, emphasising the importance of local capillary supply in promoting exercise tolerance.
Keywords: angiogenesis; exercise; metabolomics; muscle stimulation.
© 2023 The Authors. Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare no conflict of interests.
Figures








References
-
- Agudelo, L. Z. , Ferreira, D. M. , Dadvar, S. , Cervenka, I. , Ketscher, L. , Izadi, M. , Zhengye, L. , Furrer, R. , Handschin, C. , & Venckunas, T. (2019). Skeletal muscle PGC‐1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue‐resistance. Nature Communications, 10(1), 1–12. - PMC - PubMed
-
- Allison, D. J. , Nederveen, J. P. , Snijders, T. , Bell, K. E. , Kumbhare, D. , Phillips, S. M. , Parise, G. , & Heisz, J. J. (2019). Exercise training impacts skeletal muscle gene expression related to the kynurenine pathway. American Journal of Physiology. Cell Physiology, 316(3), C444–C448. - PMC - PubMed
-
- Al‐Shammari, A. A. , Gaffney, E. A. , & Egginton, S. (2014). Modelling capillary oxygen supply capacity in mixed muscles: Capillary domains revisited. Journal of Theoretical Biology, 356, 47–61. - PubMed
-
- Al‐Shammari, A. A. , Kissane, R. W. P. , Holbek, S. , Mackey, A. L. , Andersen, T. R. , Gaffney, E. A. , Kjaer, M. , & Egginton, S. (2019). Integrated method for quantitative morphometry and oxygen transport modeling in striated muscle. Journal of Applied Physiology, 126(3), 544–557. - PubMed
-
- Annex, B. H. , Torgan, C. E. , Lin, P. , Taylor, D. A. , Thompson, M. A. , Peters, K. G. , & Kraus, W. E. (1998). Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. American Journal of Physiology. Heart and Circulatory Physiology, 274(3), H860–H867. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

