A fixed-duration immunochemotherapy approach in CLL: 5.5-year results from the phase 2 ICLL-07 FILO trial
- PMID: 37026799
- PMCID: PMC10410135
- DOI: 10.1182/bloodadvances.2022009594
A fixed-duration immunochemotherapy approach in CLL: 5.5-year results from the phase 2 ICLL-07 FILO trial
Abstract
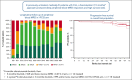
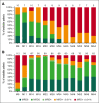
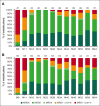
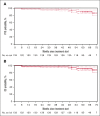
In previously untreated, medically fit patients with chronic lymphocytic leukemia (CLL), research is focused on developing fixed-duration strategies to improve long-term outcomes while sparing patients from serious toxicities. The ICLL-07 trial evaluated a fixed-duration (15-month) immunochemotherapy approach in which after obinutuzumab-ibrutinib induction for 9 months, patients (n = 10) in complete remission (CR) with bone marrow (BM) measurable residual disease (MRD) <0.01% continued only ibrutinib 420 mg/day for 6 additional months (I arm), whereas the majority (n = 115) received up to 4 cycles of fludarabine/cyclophosphamide-obinutuzumab 1000 mg alongside the ibrutinib (I-FCG arm). Primary analysis at month 16 showed that 84 of 135 (62.2%) patients enrolled achieved CR with a BM MRD <0.01%. Here, we report follow-up at median 63 months. Peripheral blood (PB) MRD was assessed 6 monthly beyond the end of treatment using a highly sensitive (10-6) flow cytometry technique. In the I-FCG arm, the PB MRD <0.01% rate (low-level positive <0.01% or undetectable with limit of detection ≤10-4) in evaluable patients was still 92.5% (74/80) at month 40 and 80.6% (50/62) at month 64. No differences in the PB MRD status were apparent per to the IGHV mutational status. In the overall population, 4-year progression-free and overall survival rates were 95.5% and 96.2%, respectively. Twelve deaths occurred overall. Fourteen serious adverse events occurred beyond the end of treatment. Thus, our fixed-duration immunochemotherapy approach produced deep and sustained PB MRD responses, high survival rates, and low long-term toxicity. A randomized trial is needed to compare our immunochemotherapy approach with a chemotherapy-free strategy. This trial was registered at www.clinicaltrials.gov as #NCT02666898.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: R.L. reports personal fees from AbbVie and AstraZeneca; nonfinancial support from Janssen; and personal fees and nonfinancial support from Alexion. M.L.G.-T. reports personal fees from Alexion Pharma France. M.-S.D. reports personal fees and nonfinancial support from Janssen and AbbVie. K.L. reports grants from Takeda; personal fees from Amgen, Gilead, and Janssen; and grants and personal fees from AbbVie, Novartis, Roche, and Sandoz. E.F. reports personal fees and nonfinancial support from AbbVie, Janssen, and AstraZeneca. O.T. reports personal fees from Takeda, and personal fees and nonfinancial support from AbbVie, Gilead, Janssen, Roche, and Sandoz. A.D. reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. V. Leblond reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen and Roche. P.C. reports personal fees from Novartis and Roche; nonfinancial support from Gilead and Sandoz; and personal fees and nonfinancial support from Takeda. G.C. reports personal fees from Celgene, Gilead, Janssen, and Roche. L.M.F. reports personal fees from Gilead, Janssen, Roche, and Takeda. L.Y. reports grants from AbbVie, Janssen, Gilead, and Roche. C.D. reports personal fees from Janssen; nonfinancial support from Gilead; and personal fees and nonfinancial support from AbbVie and Roche. F.C. reports grants and personal fees from Sunesis, and personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. V. Lévy reports personal fees from AbbVie, Gilead, Janssen, and Roche. P.F. reports personal fees and nonfinancial support from AbbVie, Gilead, Janssen, and Roche. The remaining authors declare no competing interests.
Figures
References
-
- Thompson PA. SOHO state of the art updates and next questions: BTK inhibitors combined with chemoimmunotherapy in CLL, the best of both worlds. Clin Lymphoma Myeloma Leuk. 2022;22(4):205–209. - PubMed
-
- Hallek M, Fürstenau M. How to approach CLL in clinical practice. Hematol Oncol. 2019;37(suppl 1):38–42. - PubMed
-
- Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

