TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study
- PMID: 37026861
- PMCID: PMC10389515
- DOI: 10.1097/JS9.0000000000000256
TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study
Abstract
Background: The long-term survival of patients with hepatocellular carcinoma (HCC) with portal vein tumour thrombus (PVTT) is poor. Systemic therapy, transcatheter arterial chemoembolization (TACE), and hepatic artery infusion chemotherapy are widely used in HCC patients with PVTT. This study aims to explore the efficacy of combining systemic therapy with transarterial-based therapy in HCC patients with PVTT.
Materials and methods: The authors retrospectively reviewed data of HCC patients with PVTT treated with combination therapy (TACE-hepatic artery infusion chemotherapy with tyrosine kinase inhibitors and PD-1 inhibitors) or TACE alone in SYSUCC from 2011 to 2020. The overall survival (OS), progression-free survival, and overall response rate were compared. Propensity score matching was used to minimize confounding bias.
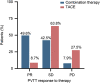
Results: A total of 743 HCC patients with PVTT received combination therapy ( n =139) or TACE alone ( n =604). After propensity score matching, the overall response rate was significantly higher in the combination group than in the TACE group [42.1% vs. 5.0%, P < 0.001 (response evaluation criteria in solid tumours); 53.7% vs. 7.8%, P < 0.001 (modified response evaluation criteria in solid tumours)]. The combination group showed significantly better OS than the TACE group (median OS not reached vs. 10.4 months, P < 0.001). The median progression-free survival of the combination and TACE groups was 14.8 and 2.3 months ( P < 0.001), respectively. Tumour downstaging followed by salvage liver resection was significantly more common for the combination therapy group than for TACE group (46.3% vs. 4.5%, P < 0.001). After salvage liver resection, 31.6% (30/95) and 1.7% (3/179) of the patients achieved a pathological complete response in the combination and TACE groups, respectively ( P < 0.001). The grade 3/4 adverse events rates were similar between the two groups (28.1% vs. 35.9%, P =0.092).
Conclusion: Compared with TACE alone, combination therapy was safe enough and resulted in survival benefits. This is a promising treatment option for HCC patients with PVTT.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare that they have no competing interests.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Lu J, Zhang XP, Zhong BY, et al. . Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol 2019;4:721–730. - PubMed
-
- Forner A, Llovert J, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–1255. - PubMed
-
- Kudo M, Finn RS, Qin S, et al. . Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173. - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. . Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020;382:1894–1905. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

