Exclusion of older adults and immunocompromised individuals in influenza, pneumococcal and COVID-19 vaccine trials before and after the COVID-19 pandemic
- PMID: 37027085
- PMCID: PMC10080508
- DOI: 10.1007/s40520-023-02380-4
Exclusion of older adults and immunocompromised individuals in influenza, pneumococcal and COVID-19 vaccine trials before and after the COVID-19 pandemic
Abstract
Background: Older adults and immunocompromised individulas are often excluded from vaccine trials.
Aim: We hypothesised that during the coronavirus disease 2019 (COVID-19) pandemic, the proportion of trials excluding these patients decreased.
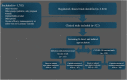
Methods: Using the US Food and Drug Administration and and European Medicines Agency search engines, we identified all vaccines approved against pneumococcal disease, influenza (quadrivalent vaccines), and COVID-19 from 2011 to 2021. Study protocols were screened for direct and indirect age exclusion criteria and exclusion of immunocompromised individuals. In addition, we reviewed the studies with no explicit exclusion criteria and investigated the actual inclusion of those individuals.
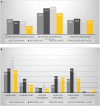
Results: We identified 2024 trial records; 1702 were excluded (e.g., use of other vaccine or risk group); and 322 studies were eligible for our review. Among the pneumococcal and influenza vaccine trials (n = 193), 81 (42%) had an explicit direct age exclusion, and 150 (78%) had an indirect age-related exclusion. In total, 163 trials (84%) trials were likely to exclude older adults. Among the COVID-19 vaccine trials (n = 129), 33 (26%) had direct age exclusion and 82 (64%) had indirect age exclusion; in total, 85 (66%) trials were likely to exclude older adults. Therefore was a 18% decrease in the proportion of trials with age-related exclusion between 2011 and 2021 (only influenza and pneumococcal vaccine trials) and 2020-2021 (only COVID-19 vaccine trials) (p = 0.014). In a sub-analysis assessing observational and randomised trials, the decrease was 25% and 9%, respectively. Immunocompromised individuals were included in 87 (45%) of the pneumococcal and influenza vaccine trials compared with 54 (42%) of the COVID-19 vaccine trials (p = 0.058).
Conclusions: During the COVID-19 pandemic, we found a decrease in the exclusion of older adults from vaccine trials but no significant change in the inclusion of immunocompromised individulas.
Keywords: COVID-19 vaccine; Exclusion; Immunocompromised; Influenza vaccine; Older adults; Pneumococcal vaccine; Vaccine trials.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest. Some of the results of this study were presented at the ESCMID educational workshop on “Vaccination of the elderly in COVID-19 times”, held on September 12–13, 2022, in Venice, Italy.
Figures

References
-
- Warren-Gash C, Perel P, Guthrie B, Mercer SW, Clark A, Jit M, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8:e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

