High-resolution imaging of the excised porcine heart at a whole-body 7 T MRI system using an 8Tx/16Rx pTx coil
- PMID: 37027119
- PMCID: PMC10140105
- DOI: 10.1007/s10334-023-01077-z
High-resolution imaging of the excised porcine heart at a whole-body 7 T MRI system using an 8Tx/16Rx pTx coil
Abstract
Introduction: MRI of excised hearts at ultra-high field strengths ([Formula: see text]≥7 T) can provide high-resolution, high-fidelity ground truth data for biomedical studies, imaging science, and artificial intelligence. In this study, we demonstrate the capabilities of a custom-built, multiple-element transceiver array customized for high-resolution imaging of excised hearts.
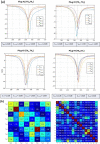
Method: A dedicated 16-element transceiver loop array was implemented for operation in parallel transmit (pTx) mode (8Tx/16Rx) of a clinical whole-body 7 T MRI system. The initial adjustment of the array was performed using full-wave 3D-electromagnetic simulation with subsequent final fine-tuning on the bench.
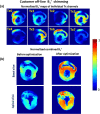
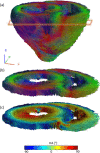
Results: We report the results of testing the implemented array in tissue-mimicking liquid phantoms and excised porcine hearts. The array demonstrated high efficiency of parallel transmits characteristics enabling efficient pTX-based B1+-shimming.
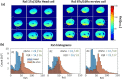
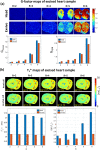
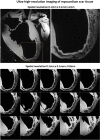
Conclusion: The receive sensitivity and parallel imaging capability of the dedicated coil were superior to that of a commercial 1Tx/32Rx head coil in both SNR and T2*-mapping. The array was successfully tested to acquire ultra-high-resolution (0.1 × 0.1 × 0.8 mm voxel) images of post-infarction scar tissue. High-resolution (isotropic 1.6 mm3 voxel) diffusion tensor imaging-based tractography provided high-resolution information about normal myocardial fiber orientation.
Keywords: Excised heart; Parallel transmit; RF-array; Ultra-high-field.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
A novel antisymmetric 16-element transceiver dipole antenna array for parallel transmit cardiac MRI in pigs at 7 T.NMR Biomed. 2022 Aug;35(8):e4726. doi: 10.1002/nbm.4726. Epub 2022 Mar 31. NMR Biomed. 2022. PMID: 35277907
-
A Novel Mono-surface Antisymmetric 8Tx/16Rx Coil Array for Parallel Transmit Cardiac MRI in Pigs at 7T.Sci Rep. 2020 Feb 20;10(1):3117. doi: 10.1038/s41598-020-59949-6. Sci Rep. 2020. PMID: 32080274 Free PMC article.
-
Design of a novel antisymmetric coil array for parallel transmit cardiac MRI in pigs at 7 T.J Magn Reson. 2019 Aug;305:195-208. doi: 10.1016/j.jmr.2019.07.004. Epub 2019 Jul 5. J Magn Reson. 2019. PMID: 31306985
-
Ultra-high field MRI: parallel-transmit arrays and RF pulse design.Phys Med Biol. 2023 Jan 18;68(2). doi: 10.1088/1361-6560/aca4b7. Phys Med Biol. 2023. PMID: 36410046 Review.
-
A Review of Parallel Transmit Arrays for Ultra-High Field MR Imaging.IEEE Rev Biomed Eng. 2024;17:351-368. doi: 10.1109/RBME.2023.3244132. Epub 2024 Jan 12. IEEE Rev Biomed Eng. 2024. PMID: 37022919
Cited by
-
Ultra-high field cardiac MRI in large animals and humans for translational cardiovascular research.Front Cardiovasc Med. 2023 May 15;10:1068390. doi: 10.3389/fcvm.2023.1068390. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37255709 Free PMC article.
References
-
- Xin SX, Huang QH, Gao Y, Li BG, Xu YK, Chen WF. Fetus MRI at 7 T: B-1 shimming strategy and SAR safety implications. IEEE Trans Microw Theory Tech. 2013;61(5):2146–2152. doi: 10.1109/TMTT.2013.2247053. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

