Local analgesia of electroacupuncture is mediated by the recruitment of neutrophils and released β-endorphins
- PMID: 37027145
- PMCID: PMC10436362
- DOI: 10.1097/j.pain.0000000000002892
Local analgesia of electroacupuncture is mediated by the recruitment of neutrophils and released β-endorphins
Abstract
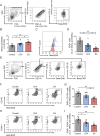
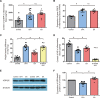
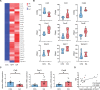
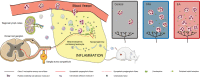
The efficacy of acupuncture in treating pain diseases has been recognized in clinical practice, and its mechanism of action has been a hot topic in academic acupuncture research. Previous basic research on acupuncture analgesia has focused mostly on the nervous system, with few studies addressing the immune system as a potential pathway of acupuncture analgesia. In this study, we investigated the effect of electroacupuncture (EA) on the β-endorphins (β-END) content, END-containing leukocyte type and number, sympathetic neurotransmitter norepinephrine (NE), and chemokine gene expression in inflamed tissues. To induce inflammatory pain, about 200 µL of complete Frester adjuvant (CFA) was injected into the unilateral medial femoral muscle of adult Wistar rats. Electroacupuncture treatment was performed for 3 days beginning on day 4 after CFA injection, with parameters of 2/100 Hz, 2 mA, and 30 minutes per treatment. The weight-bearing experiment and enzyme-linked immunosorbent assay showed that EA treatment significantly relieved spontaneous pain-like behaviors and increased the level of β-END in inflamed tissue. Injection of anti-END antibody in inflamed tissue blocked this analgesic effect. Flow cytometry and immunofluorescence staining revealed that the EA-induced increase in β-END was derived from opioid-containing ICAM-1 + /CD11b + immune cells in inflamed tissue. In addition, EA treatment increased the NE content and expression of β2 adrenergic receptor (ADR-β2) in inflammatory tissues and upregulated Cxcl1 and Cxcl6 gene expression levels. These findings provide new evidence for the peripheral analgesic effect of acupuncture treatment by recruiting β-END-containing ICAM-1 + /CD11b + immune cells and increasing the β-END content at the site of inflammation.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflict of interest to declare.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


References
-
- Binder W, Mousa SA, Sitte N, Kaiser M, Stein C, Schäfer M. Sympathetic activation triggers endogenous opioid release and analgesia within peripheral inflamed tissue. Eur J Neurosci 2004;20:92–100. - PubMed
-
- Bing Z, Villanueva L, Le Bars D. Acupuncture and diffuse noxious inhibitory controls: naloxone-reversible depression of activities of trigeminal convergent neurons. Neuroscience 1990;37:809–18. - PubMed
-
- Brack A, Labuz D, Schiltz A, Rittner HL, Machelska H, Schafer M, Reszka R, Stein C. Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology 2004;101:204–11. - PubMed
-
- Cabot PJ, Carter L, Schafer M, Stein C. Methionine-enkephalin-and Dynorphin A-release from immune cells and control of inflammatory pain. PAIN 2001;93:207–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

