A compensatory RNase E variation increases Iron Piracy and Virulence in multidrug-resistant Pseudomonas aeruginosa during Macrophage infection
- PMID: 37027441
- PMCID: PMC10115287
- DOI: 10.1371/journal.ppat.1010942
A compensatory RNase E variation increases Iron Piracy and Virulence in multidrug-resistant Pseudomonas aeruginosa during Macrophage infection
Abstract
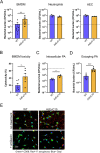
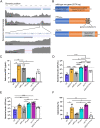
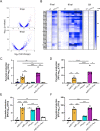
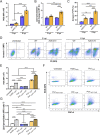
During chronic cystic fibrosis (CF) infections, evolved Pseudomonas aeruginosa antibiotic resistance is linked to increased pulmonary exacerbations, decreased lung function, and hospitalizations. However, the virulence mechanisms underlying worse outcomes caused by antibiotic resistant infections are poorly understood. Here, we investigated evolved aztreonam resistant P. aeruginosa virulence mechanisms. Using a macrophage infection model combined with genomic and transcriptomic analyses, we show that a compensatory mutation in the rne gene, encoding RNase E, increased pyoverdine and pyochelin siderophore gene expression, causing macrophage ferroptosis and lysis. We show that iron-bound pyochelin was sufficient to cause macrophage ferroptosis and lysis, however, apo-pyochelin, iron-bound pyoverdine, or apo-pyoverdine were insufficient to kill macrophages. Macrophage killing could be eliminated by treatment with the iron mimetic gallium. RNase E variants were abundant in clinical isolates, and CF sputum gene expression data show that clinical isolates phenocopied RNase E variant functions during macrophage infection. Together these data show how P. aeruginosa RNase E variants can cause host damage via increased siderophore production and host cell ferroptosis but may also be targets for gallium precision therapy.
Copyright: © 2023 Vaillancourt et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

