The critical role of engineering in the rapid development of COVID-19 diagnostics: Lessons from the RADx Tech Test Verification Core
- PMID: 37027461
- PMCID: PMC10081837
- DOI: 10.1126/sciadv.ade4962
The critical role of engineering in the rapid development of COVID-19 diagnostics: Lessons from the RADx Tech Test Verification Core
Abstract
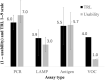
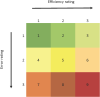
Engineering plays a critical role in the development of medical devices, and this has been magnified since 2020 as severe acute respiratory syndrome coronavirus 2 swept over the globe. In response to the coronavirus disease 2019, the National Institutes of Health launched the Rapid Acceleration of Diagnostics (RADx) initiative to help meet the testing needs of the United States and effectively manage the pandemic. As the Engineering and Human Factors team for the RADx Tech Test Verification Core, we directly assessed more than 30 technologies that ultimately contributed to an increase of the country's total testing capacity by 1.7 billion tests to date. In this review, we present central lessons learned from this "apples-to-apples" comparison of novel, rapidly developed diagnostic devices. Overall, the evaluation framework and lessons learned presented in this review may serve as a blueprint for engineers developing point-of-care diagnostics, leaving us better prepared to respond to the next global public health crisis rapidly and effectively.
Figures



Similar articles
-
The RADxSM Tech Process: Accelerating Innovation for COVID-19 Testing.IEEE Pulse. 2021 May-Jun;12(3):21-23. doi: 10.1109/MPULS.2021.3078602. IEEE Pulse. 2021. PMID: 34156930
-
The RADx Tech Clinical Studies Core: A Model for Academic Based Clinical Studies.IEEE Open J Eng Med Biol. 2021 Apr 28;2:152-157. doi: 10.1109/OJEMB.2021.3070830. eCollection 2021. IEEE Open J Eng Med Biol. 2021. PMID: 34192287 Free PMC article.
-
RADx-UP Testing Core: Access to COVID-19 Diagnostics in Community-Engaged Research with Underserved Populations.J Clin Microbiol. 2023 Aug 23;61(8):e0036723. doi: 10.1128/jcm.00367-23. Epub 2023 Jul 3. J Clin Microbiol. 2023. PMID: 37395655 Free PMC article.
-
Pivoting Novel Exosome-Based Technologies for the Detection of SARS-CoV-2.Viruses. 2022 May 18;14(5):1083. doi: 10.3390/v14051083. Viruses. 2022. PMID: 35632824 Free PMC article. Review.
-
Standardizing, harmonizing, and protecting data collection to broaden the impact of COVID-19 research: the rapid acceleration of diagnostics-underserved populations (RADx-UP) initiative.J Am Med Inform Assoc. 2022 Aug 16;29(9):1480-1488. doi: 10.1093/jamia/ocac097. J Am Med Inform Assoc. 2022. PMID: 35678579 Free PMC article. Review.
Cited by
-
Accelerated Development of a COVID-19 Lateral Flow Test in an Academic Setting: Lessons Learned.Acc Chem Res. 2024 May 7;57(9):1372-1383. doi: 10.1021/acs.accounts.4c00075. Epub 2024 Apr 8. Acc Chem Res. 2024. PMID: 38590049 Free PMC article.
-
Biomedical engineering and interdisciplinary research in shaping tomorrow's medicine.iScience. 2025 Jul 2;28(7):112884. doi: 10.1016/j.isci.2025.112884. eCollection 2025 Jul 18. iScience. 2025. PMID: 40687809 Free PMC article.
-
Saliva Has High Sensitivity and Specificity for Detecting SARS-CoV-2 Compared to Nasal Swabs but Exhibits Different Viral Dynamics from Days of Symptom Onset.Diagnostics (Basel). 2025 Jul 30;15(15):1918. doi: 10.3390/diagnostics15151918. Diagnostics (Basel). 2025. PMID: 40804882 Free PMC article.
-
A capacitive laser-induced graphene based aptasensor for SARS-CoV-2 detection in human saliva.PLoS One. 2023 Aug 17;18(8):e0290256. doi: 10.1371/journal.pone.0290256. eCollection 2023. PLoS One. 2023. PMID: 37590297 Free PMC article.
-
Evaluation of the analytical performance of the 15-minute point-of-care DASH™ SARS-CoV-2 RT-qPCR test.Diagn Microbiol Infect Dis. 2024 Jan;108(1):116120. doi: 10.1016/j.diagmicrobio.2023.116120. Epub 2023 Oct 21. Diagn Microbiol Infect Dis. 2024. PMID: 37898036 Free PMC article.
References
-
- P. E. George, C. L. Stokes, L. C. Bassit, A. Chahroudi, J. Figueroa, M. A. Griffiths, S. Heilman, D. N. Ku, E. J. Nehl, T. Leong, J. M. Levy, R. Kempker, R. G. Mannino, M. Mavigner, S. I. Park, A. Rao, P. A. Rebolledo, J. D. Roback, B. B. Rogers, R. F. Schinazi, J. Sullivan, E. A. Tyburski, M. B. Vos, J. J. Waggoner, Y. F. Wang, J. Madsen, D. S. Wechsler, C. H. Joiner, G. S. Martin, W. A. Lam, Covid-19 will not "magically disappear": Why access to widespread testing is paramount. Am. J. Hematol. 96, 174–178 (2021). - PMC - PubMed
-
- T. Struyf, J. J. Deeks, J. Dinnes, Y. Takwoingi, C. Davenport, M. M. Leeflang, R. Spijker, L. Hooft, D. Emperador, J. Domen, A. Tans, S. Janssens, D. Wickramasinghe, V. Lannoy, S. R. A. Horn, A. Van den Bruel; Cochrane COVID-19 Diagnostic Test Accuracy Group 3 , Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database Syst. Rev. 5, CD013665 (2022). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

