History and current status of clinical studies using human pluripotent stem cells
- PMID: 37028422
- PMCID: PMC10444540
- DOI: 10.1016/j.stemcr.2023.03.005
History and current status of clinical studies using human pluripotent stem cells
Abstract
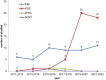
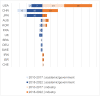
The Human Pluripotent Stem Cell Registry established a database of clinical studies using human pluripotent stem cells (PSCs) as starting material for cell therapies. Since 2018, we have observed a switch toward human induced pluripotent stem cells (iPSCs) from human embryonic stem cells. However, rather than using iPSCs for personalized medicines, allogeneic approaches dominate. Most treatments target ophthalmopathies, and genetically modified iPSCs are used to generate tailored cells. We observe a lack of standardization and transparency about the PSCs lines used, characterization of the PSC-derived cells, and the preclinical models and assays applied to show efficacy and safety.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests The authors declare no competing interests.
Figures

References
-
- DeVito N.J., Bacon S., Goldacre B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study. Lancet. 2020;395:361–369. doi: 10.1016/S0140-6736(19)33220-9. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

