Persistence of immune response in heterologous COVID vaccination schedules in the Com-COV2 study - A single-blind, randomised trial incorporating mRNA, viral-vector and protein-adjuvant vaccines
- PMID: 37028454
- PMCID: PMC10076082
- DOI: 10.1016/j.jinf.2023.03.027
Persistence of immune response in heterologous COVID vaccination schedules in the Com-COV2 study - A single-blind, randomised trial incorporating mRNA, viral-vector and protein-adjuvant vaccines
Abstract
Background: Heterologous COVID vaccine priming schedules are immunogenic and effective. This report aims to understand the persistence of immune response to the viral vectored, mRNA and protein-based COVID-19 vaccine platforms used in homologous and heterologous priming combinations, which will inform the choice of vaccine platform in future vaccine development.
Methods: Com-COV2 was a single-blinded trial in which adults ≥ 50 years, previously immunised with single dose 'ChAd' (ChAdOx1 nCoV-19, AZD1222, Vaxzevria, Astrazeneca) or 'BNT' (BNT162b2, tozinameran, Comirnaty, Pfizer/BioNTech), were randomised 1:1:1 to receive a second dose 8-12 weeks later with either the homologous vaccine, or 'Mod' (mRNA-1273, Spikevax, Moderna) or 'NVX' (NVX-CoV2373, Nuvaxovid, Novavax). Immunological follow-up and the secondary objective of safety monitoring were performed over nine months. Analyses of antibody and cellular assays were performed on an intention-to-treat population without evidence of COVID-19 infection at baseline or for the trial duration.
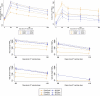
Findings: In April/May 2021, 1072 participants were enrolled at a median of 9.4 weeks after receipt of a single dose of ChAd (N = 540, 45% female) or BNT (N = 532, 39% female) as part of the national vaccination programme. In ChAd-primed participants, ChAd/Mod had the highest anti-spike IgG from day 28 through to 6 months, although the heterologous vs homologous geometric mean ratio (GMR) dropped from 9.7 (95% CI (confidence interval): 8.2, 11.5) at D28 to 6.2 (95% CI: 5.0, 7.7) at D196. The heterologous/homologous GMR for ChAd/NVX similarly dropped from 3.0 (95% CI:2.5,3.5) to 2.4 (95% CI:1.9, 3.0). In BNT-primed participants, decay was similar between heterologous and homologous schedules with BNT/Mod inducing the highest anti-spike IgG for the duration of follow-up. The adjusted GMR (aGMR) for BNT/Mod compared with BNT/BNT increased from 1.36 (95% CI: 1.17, 1.58) at D28 to 1.52 (95% CI: 1.21, 1.90) at D196, whilst for BNT/NVX this aGMR was 0.55 (95% CI: 0.47, 0.64) at day 28 and 0.62 (95% CI: 0.49, 0.78) at day 196. Heterologous ChAd-primed schedules produced and maintained the largest T-cell responses until D196. Immunisation with BNT/NVX generated a qualitatively different antibody response to BNT/BNT, with the total IgG significantly lower than BNT/BNT during all follow-up time points, but similar levels of neutralising antibodies.
Interpretation: Heterologous ChAd-primed schedules remain more immunogenic over time in comparison to ChAd/ChAd. BNT-primed schedules with a second dose of either mRNA vaccine also remain more immunogenic over time in comparison to BNT/NVX. The emerging data on mixed schedules using the novel vaccine platforms deployed in the COVID-19 pandemic, suggest that heterologous priming schedules might be considered as a viable option sooner in future pandemics.
Isrctn: 27841311 EudraCT:2021-001275-16.
Keywords: Adenovirus vector; Antibody; Heterologous; Persistence; SARS-CoV2; T-cell; Vaccine; mRNA lipid nanoparticle.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests At the time of this study, MDS acted on behalf of the University of Oxford as an Investigator on studies funded or sponsored by vaccine manufacturers including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM vaccines. He received no personal financial payment for this work. Subsequent to this study MDS is employed by Moderna Biotech UK and holds equity in this company. Moderna Biotech had no role in the study design, analysis of data or interpretation of results. JSN-V-T was seconded to the Department of Health and Social Care (DHSC), England from October 2017 to March 2022; since leaving DHSC he reports a lecture fee from AstraZeneca. AMC and DMF are investigators on studies funded by Pfizer and Unilever. They receive no personal financial payment for this work. AF is a member of the Joint Committee on Vaccination and Immunisation and chair of the WHO European Technical Advisory Group of Experts (ETAGE) on Immunisation. He is an investigator and/or provides consultative advice on clinical trials and studies of COVID-19 vaccines produced by AstraZeneca, Janssen, Valneva, Pfizer, and Sanofi, and of other vaccines from these and other manufacturers, including GlaxoSmithKline, VPI Pharmaceuticals, Takeda, and Bionet Asia. He receives no personal remuneration or benefits for any of this work. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator and/or providing consultative advice on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck, and Valneva vaccines and antimicrobials. He receives no personal financial payment for this work. PTH acts on behalf of St. George's University of London as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, and Valneva. He receives no personal financial payment for this work. CAG acts on behalf of University Hospitals Birmingham NHS Foundation Trust as an investigator on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, CureVac, Moderna, and Valneva. He receives no personal financial payment for this work. VL acts on behalf of University College London Hospitals NHS Foundation Trust as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers including Pfizer, AstraZeneca, and Valneva. He receives no personal financial payment for this work. TL is named as an inventor on a patent application covering the ChAd vaccine and is an occasional consultant to Vaccitech, unrelated to this work. ALG is named as an inventor on a patent covering use of a particular promoter construct that is often used in -vectored vaccines and is incorporated in the ChAdOx1 nCoV-19 vaccine. ALG may benefit from royalty income paid to the University of Oxford from sales of this vaccine by AstraZeneca and its sublicensees under the University’s revenue sharing policy. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. All other authors declare no competing interests. The views expressed in this manuscript are those of its authors and not necessarily those of DHSC, VTF or NIHR.
Figures




Similar articles
-
Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial.Lancet. 2022 Jan 1;399(10319):36-49. doi: 10.1016/S0140-6736(21)02718-5. Epub 2021 Dec 6. Lancet. 2022. PMID: 34883053 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial.Lancet. 2021 Sep 4;398(10303):856-869. doi: 10.1016/S0140-6736(21)01694-9. Epub 2021 Aug 6. Lancet. 2021. PMID: 34370971 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial.Lancet. 2021 Dec 18;398(10318):2258-2276. doi: 10.1016/S0140-6736(21)02717-3. Epub 2021 Dec 2. Lancet. 2021. PMID: 34863358 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine: a systematic review.Infect Dis Poverty. 2022 May 13;11(1):53. doi: 10.1186/s40249-022-00977-x. Infect Dis Poverty. 2022. PMID: 35562753 Free PMC article.
-
To mix or not to mix? A rapid systematic review of heterologous prime-boost covid-19 vaccination.Expert Rev Vaccines. 2021 Oct;20(10):1211-1220. doi: 10.1080/14760584.2021.1971522. Epub 2021 Sep 1. Expert Rev Vaccines. 2021. PMID: 34415818 Free PMC article.
Cited by
-
Impact of Repeated Variant Exposures on Cellular and Humoral Immunogenicity Induced by SARS-CoV-2 Vaccines.Vaccines (Basel). 2024 Dec 13;12(12):1408. doi: 10.3390/vaccines12121408. Vaccines (Basel). 2024. PMID: 39772069 Free PMC article.
-
Exploring How Adipose Tissue, Obesity, and Gender Influence the Immune Response to Vaccines: A Comprehensive Narrative Review.Int J Mol Sci. 2025 Jan 20;26(2):862. doi: 10.3390/ijms26020862. Int J Mol Sci. 2025. PMID: 39859575 Free PMC article. Review.
-
T and B cell responses in different immunization scenarios for COVID-19: a narrative review.Front Immunol. 2025 Mar 18;16:1535014. doi: 10.3389/fimmu.2025.1535014. eCollection 2025. Front Immunol. 2025. PMID: 40170841 Free PMC article. Review.
-
A short peptide for efficient cellular mRNA delivery: A potential application for inducing an immune response.Mol Ther Nucleic Acids. 2025 Jul 29;36(3):102650. doi: 10.1016/j.omtn.2025.102650. eCollection 2025 Sep 9. Mol Ther Nucleic Acids. 2025. PMID: 40832628 Free PMC article.
-
Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine.Vaccines (Basel). 2023 Nov 15;11(11):1720. doi: 10.3390/vaccines11111720. Vaccines (Basel). 2023. PMID: 38006052 Free PMC article.
References
-
- Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis [Internet]. Vol. 22(no. 9); 2022 [cited 2022 Oct 3], p. 1293–302. Available from: 〈http://www.thelancet.com/article/S1473309922003206/fulltext〉. - PMC - PubMed
-
- Our World in Data Coronavirus (COVID-19) vaccinations – statistics and research [internet] Our World Data. 2021 [[cited 2021 Jun 20]. Available from: 〈 https://ourworldindata.org/covid-vaccinations〉]
-
- Stuart A.S., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2021 [[cited 2021 Dec 13]. Available from: 〈 http://www.thelancet.com/article/S0140673621027185/fulltext〉] - PMC - PubMed
-
- University of Oxford. COM-CoV3 Study website [Internet]; 2021 [cited 2022 Oct 23]. Available from: 〈https://comcovstudy.org.uk/about-com-cov3〉.
-
- Shaw R.H., Liu X., Stuart A.S. v, Greenland M., Aley P.K., Andrews N.J., et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: exploratory analyses of Com-COV, a randomised control trial. Lancet Respir Med. 2022 [[cited 2022 Jun 22]; Available from: /pmc/articles/PMC9179150/] - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

