Single particle cryo-EM analysis of Rickettsia conorii Sca2 reveals a formin-like core
- PMID: 37028467
- PMCID: PMC10200769
- DOI: 10.1016/j.jsb.2023.107960
Single particle cryo-EM analysis of Rickettsia conorii Sca2 reveals a formin-like core
Abstract
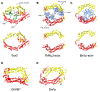
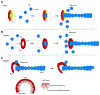
Spotted fever group Rickettsia undergo actin-based motility inside infected eukaryotic cells using Sca2 (surface cell antigen 2): an ∼ 1800 amino-acid monomeric autotransporter protein that is surface-attached to the bacterium and responsible for the assembly of long unbranched actin tails. Sca2 is the only known functional mimic of eukaryotic formins, yet it shares no sequence similarities to the latter. Using structural and biochemical approaches we have previously shown that Sca2 uses a novel actin assembly mechanism. The first ∼ 400 amino acids fold into helix-loop-helix repeats that form a crescent shape reminiscent of a formin FH2 monomer. Additionally, the N- and C- terminal halves of Sca2 display intramolecular interaction in an end-to-end manner and cooperate for actin assembly, mimicking a formin FH2 dimer. Towards a better structural understanding of this mechanism, we performed single-particle cryo-electron microscopy analysis of Sca2. While high-resolution structural details remain elusive, our model confirms the presence of a formin-like core: Sca2 indeed forms a doughnut shape, similar in diameter to a formin FH2 dimer and can accommodate two actin subunits. Extra electron density, thought to be contributed by the C-terminal repeat domain (CRD), covering one side is also observed. This structural analysis allows us to propose an updated model where nucleation proceeds by encircling two actin subunits, and elongation proceeds either by a formin-like mechanism that necessitates conformational changes in the observed Sca2 model, or via an insertional mechanism akin to that observed in the ParMRC system.
Keywords: Actin assembly; Autotransporter; FH2; Formin; Spotted fever group Rickettsia.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Rickettsia Sca2 has evolved formin-like activity through a different molecular mechanism.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):E2677-86. doi: 10.1073/pnas.1307235110. Epub 2013 Jul 1. Proc Natl Acad Sci U S A. 2013. PMID: 23818602 Free PMC article.
-
Rickettsia Sca2 Recruits Two Actin Subunits for Nucleation but Lacks WH2 Domains.Biophys J. 2019 Feb 5;116(3):540-550. doi: 10.1016/j.bpj.2018.12.009. Epub 2018 Dec 18. Biophys J. 2019. PMID: 30638962 Free PMC article.
-
Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility.Nat Cell Biol. 2010 Nov;12(11):1057-63. doi: 10.1038/ncb2109. Epub 2010 Oct 24. Nat Cell Biol. 2010. PMID: 20972427 Free PMC article.
-
Functional Mimicry of Eukaryotic Actin Assembly by Pathogen Effector Proteins.Int J Mol Sci. 2022 Oct 1;23(19):11606. doi: 10.3390/ijms231911606. Int J Mol Sci. 2022. PMID: 36232907 Free PMC article. Review.
-
FHOD proteins in actin dynamics--a formin' class of its own.Small GTPases. 2014;5(2):11. doi: 10.4161/21541248.2014.973765. Small GTPases. 2014. PMID: 25483300 Free PMC article. Review.
Cited by
-
Pathogenic Rickettsia spp. as emerging models for bacterial biology.J Bacteriol. 2024 Feb 22;206(2):e0040423. doi: 10.1128/jb.00404-23. Epub 2024 Feb 5. J Bacteriol. 2024. PMID: 38315013 Free PMC article. Review.
-
Membrane-dependent actin polymerization mediated by the Legionella pneumophila effector protein MavH.PLoS Pathog. 2023 Jul 18;19(7):e1011512. doi: 10.1371/journal.ppat.1011512. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37463171 Free PMC article.
-
Divergent Rickettsia species exhibit distinct mechanisms of actin-based motility.bioRxiv [Preprint]. 2025 Aug 22:2025.08.14.669974. doi: 10.1101/2025.08.14.669974. bioRxiv. 2025. PMID: 40894556 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

