The Dynamic Role of Endoplasmic Reticulum Stress in Chronic Liver Disease
- PMID: 37028592
- PMCID: PMC10548273
- DOI: 10.1016/j.ajpath.2023.03.009
The Dynamic Role of Endoplasmic Reticulum Stress in Chronic Liver Disease
Abstract
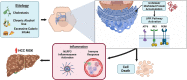
Chronic liver disease (CLD) is a major worldwide public health threat, with an estimated prevalence of 1.5 billion individuals with CLD in 2020. Chronic activation of endoplasmic reticulum (ER) stress-related pathways is recognized as substantially contributing to the pathologic progression of CLD. The ER is an intracellular organelle that folds proteins into their correct three-dimensional shapes. ER-associated enzymes and chaperone proteins highly regulate this process. Perturbations in protein folding lead to misfolded or unfolded protein accumulation in the ER lumen, resulting in ER stress and concomitant activation of the unfolded protein response (UPR). The adaptive UPR is a set of signal transduction pathways evolved in mammalian cells that attempts to reestablish ER protein homeostasis by reducing protein load and increasing ER-associated degradation. However, maladaptive UPR responses in CLD occur due to prolonged UPR activation, leading to concomitant inflammation and cell death. This review assesses the current understanding of the cellular and molecular mechanisms that regulate ER stress and the UPR in the progression of various liver diseases and the potential pharmacologic and biological interventions that target the UPR.
Copyright © 2023 American Society for Investigative Pathology. All rights reserved.
Figures


Comment in
-
The Cellular, Molecular, and Pathologic Consequences of Stress on the Liver.Am J Pathol. 2023 Oct;193(10):1353-1354. doi: 10.1016/j.ajpath.2023.07.003. Epub 2023 Aug 4. Am J Pathol. 2023. PMID: 37544504 Free PMC article. No abstract available.
References
-
- Wang M., Kaufman R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. - PubMed
-
- Ajoolabady A., Wang S., Kroemer G., Klionsky D.J., Uversky V.N., Sowers J.R., Aslkhodapasandhokmabad H., Bi Y., Ge J., Ren J. ER stress in cardiometabolic diseases: from molecular mechanisms to therapeutics. Endocr Rev. 2021;42:839–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

