Brain metastasis and survival outcomes after first-line therapy in metastatic melanoma: a multicenter DeCOG study on 1704 patients from the prospective skin cancer registry ADOREG
- PMID: 37028819
- PMCID: PMC10083858
- DOI: 10.1136/jitc-2022-005828
Brain metastasis and survival outcomes after first-line therapy in metastatic melanoma: a multicenter DeCOG study on 1704 patients from the prospective skin cancer registry ADOREG
Erratum in
-
Correction: Brain metastasis and survival outcomes after first-line therapy in metastatic melanoma: a multicenter DeCOG study on 1704 patients from the prospective skin cancer registry ADOREG.J Immunother Cancer. 2024 Jan 31;12(1):e005828corr1. doi: 10.1136/jitc-2022-005828corr1. J Immunother Cancer. 2024. PMID: 38296600 Free PMC article. No abstract available.
Abstract
Background: Despite the availability of effective systemic therapies, a significant number of advanced melanoma patients develops brain metastases. This study investigated differences in incidence and time to diagnosis of brain metastasis and survival outcomes dependent on the type of first-line therapy.
Methods: Patients with metastatic, non-resectable melanoma (AJCCv8 stage IIIC-V) without brain metastasis at start of first-line therapy (1L-therapy) were identified from the prospective multicenter real-world skin cancer registry ADOREG. Study endpoints were incidence of brain metastasis, brain metastasis-free survival (BMFS), progression-free survival (PFS), and overall survival (OS).
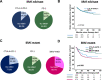
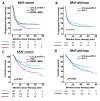
Results: Of 1704 patients, 916 were BRAF wild-type (BRAFwt) and 788 were BRAF V600 mutant (BRAFmut). Median follow-up time after start of 1L-therapy was 40.4 months. BRAFwt patients received 1L-therapy with immune checkpoint inhibitors (ICI) against CTLA-4+PD-1 (n=281) or PD-1 (n=544). In BRAFmut patients, 1L-therapy was ICI in 415 patients (CTLA-4+PD-1, n=108; PD-1, n=264), and BRAF+MEK targeted therapy (TT) in 373 patients. After 24 months, 1L-therapy with BRAF+MEK resulted in a higher incidence of brain metastasis compared with PD-1±CTLA-4 (BRAF+MEK, 30.3%; CTLA-4+PD-1, 22.2%; PD-1, 14.0%). In multivariate analysis, BRAFmut patients developed brain metastases earlier on 1L-therapy with BRAF+MEK than with PD-1±CTLA-4 (CTLA-4+PD-1: HR 0.560, 95% CI 0.332 to 0.945, p=0.030; PD-1: HR 0.575, 95% CI 0.372 to 0.888, p=0.013). Type of 1L-therapy, tumor stage, and age were independent prognostic factors for BMFS in BRAFmut patients. In BRAFwt patients, tumor stage was independently associated with longer BMFS; ECOG Performance status (ECOG-PS), lactate dehydrogenase (LDH), and tumor stage with OS. CTLA-4+PD-1 did not result in better BMFS, PFS, or OS than PD-1 in BRAFwt patients. For BRAFmut patients, multivariate Cox regression revealed ECOG-PS, type of 1L-therapy, tumor stage, and LDH as independent prognostic factors for PFS and OS. 1L-therapy with CTLA-4+PD-1 led to longer OS than PD-1 (HR 1.97, 95% CI 1.122 to 3.455, p=0.018) or BRAF+MEK (HR 2.41, 95% CI 1.432 to 4.054, p=0.001), without PD-1 being superior to BRAF+MEK.
Conclusions: In BRAFmut patients 1L-therapy with PD-1±CTLA-4 ICI resulted in a delayed and less frequent development of brain metastasis compared with BRAF+MEK TT. 1L-therapy with CTLA-4+PD-1 showed superior OS compared with PD-1 and BRAF+MEK. In BRAFwt patients, no differences in brain metastasis and survival outcomes were detected for CTLA-4+PD-1 compared with PD-1.
Keywords: melanoma.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors declare no conflicts of interest affecting this study. Conflicts of interest outside the submitted work are as following: CF has been on the advisory board or has received honoraria from Bristol Myers Squibb, Immunocore and Novartis and received travel grants from Bristol Myers Squibb, Novartis and Pierre Fabre. PM declares research support from Bristol Myers Squibb, Novartis and Merck Sharp & Dome; speakers and advisory board honoraria from Almirall Hermal, Beiersdorf, Bristol Myers Squibb, Merck Sharp & Dome, Immunocore, Merck Serono, Medac, Novartis, Pierre Fabre, Sanofi Genzyme, Sun Pharma and Roche, and travel support from Bristol Myers Squibb, Merck Sharp & Dohme, Novartis and Pierre Fabre. LB received honoraria from Amgen, Bristol-Myers Squibb and Sun Pharma. FriM has received travel support or/and speaker’s fees or/and advisor’s honoraria by Novartis, Roche, BMS, MSD and Pierre Fabre and research funding from Novartis and Roche. KK has served as consultant or/and has received honoraria from Amgen, Roche, Bristol Myers Squibb, Merck Sharp and Dohme, Pierre Fabre, and Novartis, and received travel support from Amgen, Merck Sharp and Dohme, Bristol Myers Squibb, Amgen, Pierre Fabre, Medac and Novartis. IG declares speakers and advisory board honoraria from Almirall Hermal, Bristol Myers Squibb, Merck Sharp & Dome, Novartis, Pierre Fabre, Sanofi Genzyme, Sun Pharma and Roche. RG: Invited speaker: Roche, BMS, MSD, Novartis, Amgen, Merck Serono, Almirall Hermal, SUN, Sanofi, Pierre-Fabre. Advisory board: BMS, Roche, Novartis, Almirall Hermal, MSD, Amgen, SUN, Sanofi, Pierre-Fabre, 4SC, Bayer, MerckSerono, Pfizer, Immunocore. Research grants: Novartis, Pfizer, Johnson & Johnson, Amgen, Merck-Serono, SUN Pharma, Sanofi. Travel/meeting support: Roche, BMS, SUN, Merck-Serono, Pierre-Fabre. JU is on the advisory board or has received travel support from: Amgen, BMS, GSK, Immunocore, Leo Pharma, MSD, Novartis, Pierre Fabre, Sanofi, Roche. JU has received research support from Novartis; speakers and advisory board honoraria. PT has been on the advisory board or has received honoraria from Almirall, Bristol-Myers Squibb, Novartis, Pierre-Fabre, Merck Serono, Sanofi, Roche, Kyowa Kirin, Biofrontera, and 4SC and received travel grants from Bristol Myers Squibb, and Pierre Fabre. RH reports speakers and advisory board honoraria from Bristol-Myers Squibb (BMS), Immunocore, Novartis, Pierre-Fabre, Roche and SUN pharma outside the submitted work. CP received honoraria (speaker honoraria or honoraria as a consultant) and travel support from: Novartis, BMS, Roche, Merck Serono, MSD, Celgene, AbbVie, SUNPHARMA, UCB, Allergy Therapeutics, Pierre Fabre, Kyowa Kirin and LEO. AF served as consultant to Roche, Novartis, MSD, BMS, Pierre-Fabre; received travel support from Roche, Novartis, BMS, Pierre-Fabre, received speaker fees from Roche, Novartis, BMS, MSD and CeGaT, outside the submitted work. She reports institutional research grants from BMS Stiftung Immunonkologie. FZ declares speakers and advisory board honoraria and/or travel support from BMS, MSD, Roche, Novartis, Pierre Fabre and Sanofi Aventis. FraM (Frank Meiss) served as a consultant and/or has received honoraria from Novartis, BMS, MSD, Pierre Fabre, Sanofi Genzyme, Sun Pharma and travel support from Novartis, Sun Pharma, Roche, Pierre Fabre and MSD. BS is on the advisory board or has received honoraria from Immunocore, Almirall, Pfizer, Sanofi, Novartis, Roche, BMS and MSD, research funding from Novartis and Pierre Fabre and travel support from Novartis, Roche, BMS and Pierre Fabre. AK reports receiving lecture fees and fees for serving on advisory boards from MSD Sharp & Dohme, Almirall, Infectopharm, and Boehringer Ingelheim. JW has been on the advisory board, received honoraria and/or travel grants from Bristol Myers Squibb, Novartis, MSD, and Pierre Fabre. TG has received speakers and/or advisory board honoraria and travel support from BMS, Sanofi-Genzyme, MSD, Novartis Pharma, Roche, Abbvie, Almirall, Janssen, Lilly, Pfizer, Pierre Fabre, Merck-Serono. CL has received speaker’s fees, advisory board honoraria and travel reimbursements from Merck, MSD, Roche, Almirall Hermal, Biontech, Sanofi, Sun Pharma, Kyowa Kirin, Immonocore, BMS, Pierre Fabre, Novartis. LH has received consultancy and speaker fees from Amgen, Biome Dx, BMS, Curevac, Merck, MSD, Myoncare, Novartis, Pierre-Fabre, Roche, Sanofi and SUN. SG has been on the advisory board and/or has received travel support from Bristol Myers Squibb, MSD, Sun Pharma and Novartis and received research support from Novartis and Pierre Fabre. DD has been on the advisory board or has received honoraria from BMS, Kyowa Kirin, MSD, Novartis, Pierre Fabre, Sanofi and received travel grants from Boehringer, Mylan, Pfizer. GS has received honoraria from BMS. GL has received travel support from Sun Pharma. LZ served as consultant and/or has received honoraria from Roche, Bristol-Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Novartis, Pierre Fabre, Sanofi, and Sunpharma and travel support from MSD, BMS, Amgen, Pierre Fabre, Sunpharma, Sanofi and Novartis. EL served as consultant and/or has received honoraria from Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Medac, Sanofi, Sunpharma and travel support from Medac, Bristol-Myers Squibb, Pierre Fabre, Sunpharma and Novartis. JCB received speaker’s bureau honoraria from Amgen, Pfizer, Recordati and Sanofi, and is a paid consultant/advisory board/DSMB member for Almirall, Boehringer Ingelheim, InProTher, ICON, MerckSerono, Pfizer, 4SC, and Sanofi/Regeneron. His group received research grants from Bristol-Myers Squibb, Merck Serono, HTG, IQVIA, and Alcedis. DS declares relevant financial activities (Roche, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Sanofi, Regeneron, Array, Pierre Fabre, 4SC, Helsinn, Philogen, InFlarX, Merck-Serono, SunPharma, Ultimovacs, Sandoz). SU declares research support from Bristol Myers Squibb and Merck Serono; speakers and advisory board honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Novartis and Roche, and travel support from Bristol Myers Squibb, Merck Sharp & Dohme, and Pierre Fabre.
Figures
References
-
- Dummer R, Ascierto PA, Gogas HJ, et al. . Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018;19:1315–27. 10.1016/S1470-2045(18)30497-2 - DOI - PubMed
-
- Franklin C, Mohr P, Bluhm L, et al. . Impact of radiotherapy and sequencing of systemic therapy on survival outcomes in melanoma patients with previously untreated brain metastasis: a multicenter decog study on 450 patients from the prospective skin cancer registry ADOREG. J Immunother Cancer 2022;10:e004509. 10.1136/jitc-2022-004509 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
