β-Arrestins: Structure, Function, Physiology, and Pharmacological Perspectives
- PMID: 37028945
- PMCID: PMC10441628
- DOI: 10.1124/pharmrev.121.000302
β-Arrestins: Structure, Function, Physiology, and Pharmacological Perspectives
Abstract
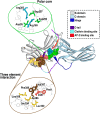
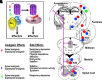
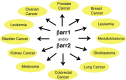
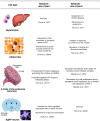
The two β-arrestins, β-arrestin-1 and -2 (systematic names: arrestin-2 and -3, respectively), are multifunctional intracellular proteins that regulate the activity of a very large number of cellular signaling pathways and physiologic functions. The two proteins were discovered for their ability to disrupt signaling via G protein-coupled receptors (GPCRs) via binding to the activated receptors. However, it is now well recognized that both β-arrestins can also act as direct modulators of numerous cellular processes via either GPCR-dependent or -independent mechanisms. Recent structural, biophysical, and biochemical studies have provided novel insights into how β-arrestins bind to activated GPCRs and downstream effector proteins. Studies with β-arrestin mutant mice have identified numerous physiologic and pathophysiological processes regulated by β-arrestin-1 and/or -2. Following a short summary of recent structural studies, this review primarily focuses on β-arrestin-regulated physiologic functions, with particular focus on the central nervous system and the roles of β-arrestins in carcinogenesis and key metabolic processes including the maintenance of glucose and energy homeostasis. This review also highlights potential therapeutic implications of these studies and discusses strategies that could prove useful for targeting specific β-arrestin-regulated signaling pathways for therapeutic purposes. SIGNIFICANCE STATEMENT: The two β-arrestins, structurally closely related intracellular proteins that are evolutionarily highly conserved, have emerged as multifunctional proteins able to regulate a vast array of cellular and physiological functions. The outcome of studies with β-arrestin mutant mice and cultured cells, complemented by novel insights into β-arrestin structure and function, should pave the way for the development of novel classes of therapeutically useful drugs capable of regulating specific β-arrestin functions.
U.S. Government work not protected by U.S. copyright.
Figures
References
-
- Aamna B, Kumar Dan A, Sahu R, Behera SK, Parida S (2022) Deciphering the signaling mechanisms of β-arrestin1 and β-arrestin2 in regulation of cancer cell cycle and metastasis. J Cell Physiol 237:3717–3733. - PubMed
-
- Abdel-Wahab OI, Levine RL (2009) Primary myelofibrosis: Update on definition, pathogenesis, and treatment. Annu Rev Med 60:233–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

