Safe drugs with high potential to block malaria transmission revealed by a spleen-mimetic screening
- PMID: 37029122
- PMCID: PMC10082216
- DOI: 10.1038/s41467-023-37359-2
Safe drugs with high potential to block malaria transmission revealed by a spleen-mimetic screening
Abstract
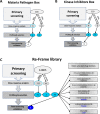
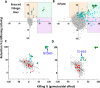
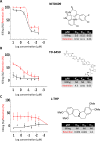
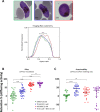
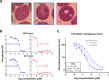
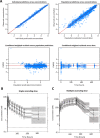
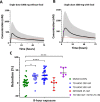
Malaria parasites like Plasmodium falciparum multiply in red blood cells (RBC), which are cleared from the bloodstream by the spleen when their deformability is altered. Drug-induced stiffening of Plasmodium falciparum-infected RBC should therefore induce their elimination from the bloodstream. Here, based on this original mechanical approach, we identify safe drugs with strong potential to block the malaria transmission. By screening 13 555 compounds with spleen-mimetic microfilters, we identified 82 that target circulating transmissible form of P. falciparum. NITD609, an orally administered PfATPase inhibitor with known effects on P. falciparum, killed and stiffened transmission stages in vitro at nanomolar concentrations. Short exposures to TD-6450, an orally-administered NS5A hepatitis C virus inhibitor, stiffened transmission parasite stages and killed asexual stages in vitro at high nanomolar concentrations. A Phase 1 study in humans with a primary safety outcome and a secondary pharmacokinetics outcome ( https://clinicaltrials.gov , ID: NCT02022306) showed no severe adverse events either with single or multiple doses. Pharmacokinetic modelling showed that these concentrations can be reached in the plasma of subjects receiving short courses of TD-6450. This physiologically relevant screen identified multiple mechanisms of action, and safe drugs with strong potential as malaria transmission-blocking agents which could be rapidly tested in clinical trials.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Splenic retention of Plasmodium falciparum gametocytes to block the transmission of malaria.Antimicrob Agents Chemother. 2015 Jul;59(7):4206-14. doi: 10.1128/AAC.05030-14. Epub 2015 May 4. Antimicrob Agents Chemother. 2015. PMID: 25941228 Free PMC article.
-
Safety, tolerability, pharmacokinetics, and antimalarial efficacy of a novel Plasmodium falciparum ATP4 inhibitor SJ733: a first-in-human and induced blood-stage malaria phase 1a/b trial.Lancet Infect Dis. 2020 Aug;20(8):964-975. doi: 10.1016/S1473-3099(19)30611-5. Epub 2020 Apr 8. Lancet Infect Dis. 2020. PMID: 32275867 Clinical Trial.
-
Multiple stiffening effects of nanoscale knobs on human red blood cells infected with Plasmodium falciparum malaria parasite.Proc Natl Acad Sci U S A. 2015 May 12;112(19):6068-73. doi: 10.1073/pnas.1505584112. Epub 2015 Apr 27. Proc Natl Acad Sci U S A. 2015. PMID: 25918423 Free PMC article.
-
The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology.Blood. 2011 Jan 13;117(2):381-92. doi: 10.1182/blood-2010-04-202911. Epub 2010 Sep 17. Blood. 2011. PMID: 20852127 Free PMC article. Review.
-
The Human Spleen in Malaria: Filter or Shelter?Trends Parasitol. 2020 May;36(5):435-446. doi: 10.1016/j.pt.2020.03.001. Epub 2020 Mar 30. Trends Parasitol. 2020. PMID: 32298631 Review.
Cited by
-
Hyperspectral analysis to assess gametocytogenesis stage progression in malaria-infected human erythrocytes.J Biomed Opt. 2025 Feb;30(2):023516. doi: 10.1117/1.JBO.30.2.023516. Epub 2025 Jan 24. J Biomed Opt. 2025. PMID: 39866361 Free PMC article.
-
The Importance of Murine Models in Determining In Vivo Pharmacokinetics, Safety, and Efficacy in Antimalarial Drug Discovery.Pharmaceuticals (Basel). 2025 Mar 18;18(3):424. doi: 10.3390/ph18030424. Pharmaceuticals (Basel). 2025. PMID: 40143200 Free PMC article. Review.
-
Multiple first-line therapeutic strategies to mitigate artemisinin resistance: cost analysis of a pilot study from a health system perspective in Kaya health district, Burkina Faso.Malar J. 2025 Aug 7;24(1):254. doi: 10.1186/s12936-025-05493-5. Malar J. 2025. PMID: 40775352 Free PMC article.
-
Clearance of pathogenic erythrocytes is maintained despite spleen dysfunction in children with sickle cell disease.Am J Hematol. 2024 Dec;99(12):2267-2278. doi: 10.1002/ajh.27481. Epub 2024 Sep 17. Am J Hematol. 2024. PMID: 39286963
-
An all-in-one pipeline for the in vitro discovery and in vivo testing of Plasmodium falciparum malaria transmission blocking drugs.Nat Commun. 2025 Jul 25;16(1):6884. doi: 10.1038/s41467-025-62014-3. Nat Commun. 2025. PMID: 40715087 Free PMC article.
References
-
- WHO. World Malaria Report 2021. https://www.who.int/teams/global-malaria-programme/reports/world-malaria... (2021).
-
- Balikagala, B. et al. Evidence of artemisinin-resistant Malaria in Africa. N. Engl. J. Med. 10.1056/NEJMoa2101746 (2021). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

