Tumour infiltrating lymphocytes and survival after adjuvant chemotherapy in patients with gastric cancer: post-hoc analysis of the CLASSIC trial
- PMID: 37029200
- PMCID: PMC10241786
- DOI: 10.1038/s41416-023-02257-3
Tumour infiltrating lymphocytes and survival after adjuvant chemotherapy in patients with gastric cancer: post-hoc analysis of the CLASSIC trial
Abstract
Background: Only a subset of gastric cancer (GC) patients with stage II-III benefits from chemotherapy after surgery. Tumour infiltrating lymphocytes per area (TIL density) has been suggested as a potential predictive biomarker of chemotherapy benefit.
Methods: We quantified TIL density in digital images of haematoxylin-eosin (HE) stained tissue using deep learning in 307 GC patients of the Yonsei Cancer Center (YCC) (193 surgery+adjuvant chemotherapy [S + C], 114 surgery alone [S]) and 629 CLASSIC trial GC patients (325 S + C and 304 S). The relationship between TIL density, disease-free survival (DFS) and clinicopathological variables was analysed.
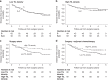
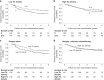
Results: YCC S patients and CLASSIC S patients with high TIL density had longer DFS than S patients with low TIL density (P = 0.007 and P = 0.013, respectively). Furthermore, CLASSIC patients with low TIL density had longer DFS if treated with S + C compared to S (P = 0.003). No significant relationship of TIL density with other clinicopathological variables was found.
Conclusion: This is the first study to suggest TIL density automatically quantified in routine HE stained tissue sections as a novel, clinically useful biomarker to identify stage II-III GC patients deriving benefit from adjuvant chemotherapy. Validation of our results in a prospective study is warranted.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
DRM is director of HeteroGenius Limited. RL received consulting fees from Astellas, Janssen, Roche, MSD not related to the current study. HIG received consulting fees from AstraZeneca and BMS not related to the current study. The remaining authors declare no competing interests.
Figures

References
-
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93. doi: 10.1200/JCO.2011.36.5908. - DOI - PubMed
-
- Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, et al. PRODIGY: a phase III study of neoadjuvant docetaxel, oxaliplatin, and S-1 plus surgery and adjuvant S-1 versus surgery and adjuvant S-1 for resectable advanced gastric cancer. J Clin Oncol. 2021;39:2903–13. doi: 10.1200/JCO.20.02914. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

