The combination of rolipram and cilostamide improved the developmental competence of cloned porcine embryos
- PMID: 37029228
- PMCID: PMC10081996
- DOI: 10.1038/s41598-023-32677-3
The combination of rolipram and cilostamide improved the developmental competence of cloned porcine embryos
Abstract
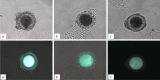
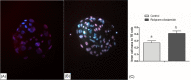
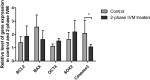
In vitro maturation of porcine oocytes is characterized by asynchronous cytoplasmic and nuclear maturation, leading to less competent oocytes supporting embryo development. The purpose of this study was to evaluate the combined effect of rolipram and cilostamide as cyclic Adenine monophosphate (cAMP) modulators to find the maximum cAMP levels that temporarily arrest meiosis. We determined the optimal time to maintain functional gap junction communication during pre-in vitro maturation to be four hours. Oocyte competence was evaluated by the level of glutathione, reactive oxygen species, meiotic progression, and gene expression. We evaluated embryonic developmental competence after parthenogenetic activation and somatic cell nuclear transfer. The combined treatment group showed significantly higher glutathione and lower reactive oxygen species levels and a higher maturation rate than the control and single treatment groups. Cleavage and blastocyst formation rates in parthenogenetic activation and somatic cell nuclear transfer embryos were higher in two-phase in vitro maturation than in the other groups. The relative levels of BMP15and GDF9 expression were increased in two-phase in vitro maturation. Somatic cell nuclear transfer blastocysts from two-phase in vitro maturation oocytes showed a lower level of expression of apoptotic genes than the control, indicating better pre-implantation developmental competence. The combination of rolipram and cilostamide resulted in optimal synchrony of cytoplasmic and nuclear maturation in porcine in vitro matured oocytes and there by enhanced the developmental competence of pre-implantation embryos.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
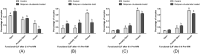



Similar articles
-
Cilostamide and forskolin treatment during pre-IVM improves preimplantation development of cloned embryos by influencing meiotic progression and gap junction communication in pigs.Theriogenology. 2016 Aug;86(3):757-65. doi: 10.1016/j.theriogenology.2016.02.029. Epub 2016 Mar 8. Theriogenology. 2016. PMID: 27056415
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Effects of manganese on maturation of porcine oocytes in vitro and their subsequent embryo development after parthenogenetic activation and somatic cell nuclear transfer.J Reprod Dev. 2019 Jun 14;65(3):259-265. doi: 10.1262/jrd.2019-001. Epub 2019 Mar 21. J Reprod Dev. 2019. PMID: 30905887 Free PMC article.
-
Supplementation with spermine during in vitro maturation of porcine oocytes improves early embryonic development after parthenogenetic activation and somatic cell nuclear transfer.J Anim Sci. 2016 Mar;94(3):963-70. doi: 10.2527/jas.2015-9761. J Anim Sci. 2016. PMID: 27065258
-
The new system of shorter porcine oocyte in vitro maturation (18 hours) using ≥8 mm follicles derived from cumulus-oocyte complexes.Theriogenology. 2014 Jan 15;81(2):291-301. doi: 10.1016/j.theriogenology.2013.09.028. Epub 2013 Oct 17. Theriogenology. 2014. PMID: 24220361
Cited by
-
BDE-47 Induces Mitochondrial Dysfunction and Endoplasmic Reticulum Stress to Inhibit Early Porcine Embryonic Development.Animals (Basel). 2023 Jul 13;13(14):2291. doi: 10.3390/ani13142291. Animals (Basel). 2023. PMID: 37508068 Free PMC article.
References
-
- Cho J, Kim G, Qamar AY, Fang X, Roy PK, Tanga BM, et al. Improved efficiencies in the generation of multigene-modified pigs by recloning and using sows as the recipient. Zygote. 2021;30:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

