Time to reimbursement of novel anticancer drugs in Europe: a case study of seven European countries
- PMID: 37030113
- PMCID: PMC10163159
- DOI: 10.1016/j.esmoop.2023.101208
Time to reimbursement of novel anticancer drugs in Europe: a case study of seven European countries
Abstract
Background: Time to reimbursement (TTR) of new anticancer medicines differs between countries and contributes to unequal access. We aimed to investigate TTR of new anticancer medicines and explore factors influencing the reimbursement process in seven high-income European countries.
Materials and methods: We carried out a retrospective case study of anticancer medicines with European Union Market Access (EU-MA) and a positive Committee for Medicinal Products for Human Use opinion from 2016 until 2021 with subsequent national reimbursement approval (NRA). The National Health Technology Assessment (HTA) and reimbursement websites of Germany, France, UK, the Netherlands, Belgium, Norway and Switzerland were used to identify TTR, defined as time from EU-MA to NRA. Additionally, we investigated medication-, country-, indication- and pharma-related factors potentially influencing TTR.
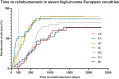
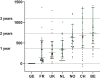
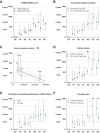
Results: Thirty-five medicines were identified for which TTR ranged from -81 days to 2320 days (median 407 days). At data cut-off, 16 (46%) were reimbursed in all seven countries. Overall, the shortest TTR was in Germany (median 3 days, all medicines reimbursed <5 days). The time limit for reimbursement of 180 days stated by the Council of European Communities after the EU-MA (EU Transparency Directive) was met for 100% of included medicines in Germany, 51% in France, 29% in the UK and the Netherlands, 14% in Switzerland, 6% in Norway and 3% in Belgium. The TTR was significantly different between countries (P < 0.001). In multivariate analysis, factors associated with shorter TTR were higher gross domestic product (GDP), absence of a pre-assessment procedure and submission by a big pharmaceutical company.
Conclusions: TTR of anticancer medicines varies significantly between seven high-income European countries and leads to inequality in access. Among explored medication-, country-, indication- and pharma-related factors we found that a high GDP, the absence of a pre-assessment procedure and submission by big pharmaceutical companies were associated with shorter TTR.
Keywords: anticancer medicines; drug access; inequality; regulatory approval; reimbursement.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Role of the funder The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Disclosure HCP and CEMH are members of the platform ‘Medicine for Society’ (see funding), CEMH reports to be involved in pre-marketing studies with Genzyme, Protalix and Idorsia. HWML declared a consultant or advisory role: Amphera, AstraZeneca, Beigene, BMS, Daiichy-Sankyo, Dragonfly, Eli Lilly, MSD, Nordic Pharma, Servier and a research funding and/or medication supply: Bayer, BMS, Celgene, Janssen, Incyte, Eli Lilly, MSD, Nordic Pharma, Philips, Roche, Servier; and a speaker role: Astellas, Benecke, Daiichy-Sankyo, JAAP, Medtalks, Novartis, Travel Congress Management B.V. All other authors have declared no conflicts of interest.
Figures

Similar articles
-
Do European regulatory measures accelerate national market access in Belgium? A retrospective analysis of medicines centrally authorised between 2015 and 2020.BMJ Open. 2025 Jan 9;15(1):e091361. doi: 10.1136/bmjopen-2024-091361. BMJ Open. 2025. PMID: 39788772 Free PMC article.
-
Health technology assessment for cancer medicines across the G7 countries and Oceania: an international, cross-sectional study.Lancet Oncol. 2023 Jun;24(6):624-635. doi: 10.1016/S1470-2045(23)00175-4. Lancet Oncol. 2023. PMID: 37269843
-
Market access to new anticancer medicines for children and adolescents with cancer in Europe.Eur J Cancer. 2022 Apr;165:146-153. doi: 10.1016/j.ejca.2022.01.034. Epub 2022 Feb 27. Eur J Cancer. 2022. PMID: 35235871
-
Achieving equal and timely access to innovative anticancer drugs in the European Union (EU): summary of a multidisciplinary CECOG-driven roundtable discussion with a focus on Eastern and South-Eastern EU countries.ESMO Open. 2019 Nov 13;4(6):e000550. doi: 10.1136/esmoopen-2019-000550. eCollection 2019. ESMO Open. 2019. PMID: 31798977 Free PMC article. Review.
-
Pricing and Reimbursement of Patent-Protected Medicines: Challenges and Lessons from South-Eastern Europe.Appl Health Econ Health Policy. 2021 Nov;19(6):915-927. doi: 10.1007/s40258-021-00678-w. Epub 2021 Sep 23. Appl Health Econ Health Policy. 2021. PMID: 34553334 Review.
Cited by
-
Demographic Analysis of Cancer Research Priorities and Treatment Correlations.Curr Oncol. 2024 Mar 29;31(4):1839-1864. doi: 10.3390/curroncol31040139. Curr Oncol. 2024. PMID: 38668042 Free PMC article.
-
The challenges of access to innovative medicines with limited evidence in the European Union.Front Pharmacol. 2023 Aug 31;14:1215431. doi: 10.3389/fphar.2023.1215431. eCollection 2023. Front Pharmacol. 2023. PMID: 37719853 Free PMC article. Review.
-
Tearing down inequalities in the healthcare system across Europe: the BEACON project.Front Public Health. 2025 Jun 5;13:1520772. doi: 10.3389/fpubh.2025.1520772. eCollection 2025. Front Public Health. 2025. PMID: 40538689 Free PMC article. Review.
-
Access in all areas? A round up of developments in market access and health technology assessment: part 1.J Comp Eff Res. 2023 Oct;12(10):e230129. doi: 10.57264/cer-2023-0129. Epub 2023 Aug 16. J Comp Eff Res. 2023. PMID: 37584405 Free PMC article.
-
Is Canada Moving towards a More Agile Regulatory Approval and Reimbursement Process with a Shifting Role for Real-World Evidence (RWE) for Oncology Drugs?Curr Oncol. 2024 Sep 18;31(9):5599-5607. doi: 10.3390/curroncol31090414. Curr Oncol. 2024. PMID: 39330042 Free PMC article.
References
-
- Transparency of decisions regulating the prices and the reimbursement of medicinal products in EU countries. Off J Eur Union. 1989:8–11.
-
- Janzic U., Knez L., Janzic A., Cufer T. Time to access to novel anticancer drugs and the correlation with ESMO-Magnitude of Clinical Benefit Scale in Slovenia. Expert Rev Pharmacoecon Outcomes Res. 2019;19(6):717–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

