The combination of immunotherapy and a glutamine metabolism inhibitor represents an effective therapeutic strategy for advanced and metastatic murine pancreatic adenocarcinoma
- PMID: 37030115
- PMCID: PMC10182763
- DOI: 10.1016/j.intimp.2023.110150
The combination of immunotherapy and a glutamine metabolism inhibitor represents an effective therapeutic strategy for advanced and metastatic murine pancreatic adenocarcinoma
Abstract
Despite constant advances in cancer research, the treatment of pancreatic adenocarcinoma remains extremely challenging. The intratumoral immunotherapy approach that was developed by our research group and was based on a combination of mannan-BAM, TLR ligands, and anti-CD40 antibody (MBTA) showed promising therapeutic effects in various murine tumor models, including a pancreatic adenocarcinoma model (Panc02). However, the efficacy of MBTA therapy in the Panc02 model was negatively correlated with tumor size at the time of therapy initiation. Here, we aimed to further improve the outcome of MBTA therapy in the Panc02 model using the glutamine antagonist 6-diazo-5-oxo-L-norleucine (DON). The combination of intratumoral MBTA therapy and intraperitoneal administration of DON resulted in the complete elimination of advanced Panc02 subcutaneous tumors (140.8 ± 46.8 mm3) in 50% of treated animals and was followed by development of long-term immune memory. In the bilateral Panc02 subcutaneous tumor model, we observed a significant reduction in tumor growth in both tumors as well as prolonged survival of treated animals. The appropriate timing and method of administration of DON were also addressed to maximize its therapeutic effects and minimize its side effects. In summary, our findings demonstrate that the intraperitoneal application of DON significantly improves the efficacy of intratumoral MBTA therapy in both advanced and bilateral Panc02 subcutaneous tumor murine models.
Keywords: Cancer; Glutamine antagonist; Immunotherapy; Intratumoral; Pancreatic adenocarcinoma.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
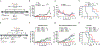

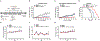
Similar articles
-
Mannan-BAM, TLR ligands, and anti-CD40 immunotherapy in established murine pancreatic adenocarcinoma: understanding therapeutic potentials and limitations.Cancer Immunol Immunother. 2021 Nov;70(11):3303-3312. doi: 10.1007/s00262-021-02920-9. Epub 2021 Apr 15. Cancer Immunol Immunother. 2021. PMID: 33855601 Free PMC article.
-
Identification of Immune Cell Infiltration in Murine Pheochromocytoma during Combined Mannan-BAM, TLR Ligand, and Anti-CD40 Antibody-Based Immunotherapy.Cancers (Basel). 2021 Aug 5;13(16):3942. doi: 10.3390/cancers13163942. Cancers (Basel). 2021. PMID: 34439097 Free PMC article.
-
Neoadjuvant intratumoral MBT(A) immunotherapy prevents distant metastases and recurrence in murine models.Cancer Lett. 2025 Mar 1;612:217464. doi: 10.1016/j.canlet.2025.217464. Epub 2025 Jan 12. Cancer Lett. 2025. PMID: 39809356
-
Mannan-BAM, TLR Ligands, Anti-CD40 Antibody (MBTA) Vaccine Immunotherapy: A Review of Current Evidence and Applications in Glioblastoma.Int J Mol Sci. 2021 Mar 26;22(7):3455. doi: 10.3390/ijms22073455. Int J Mol Sci. 2021. PMID: 33810617 Free PMC article. Review.
-
Role of B cells in intratumoral MBTA immunotherapy of murine pheochromocytoma model.Best Pract Res Clin Endocrinol Metab. 2025 Jan;39(1):101941. doi: 10.1016/j.beem.2024.101941. Epub 2024 Sep 11. Best Pract Res Clin Endocrinol Metab. 2025. PMID: 39278811 Review.
Cited by
-
A glutamine tug-of-war between cancer and immune cells: recent advances in unraveling the ongoing battle.J Exp Clin Cancer Res. 2024 Mar 8;43(1):74. doi: 10.1186/s13046-024-02994-0. J Exp Clin Cancer Res. 2024. PMID: 38459595 Free PMC article. Review.
References
-
- Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: ready for clinical translation? Gut. 2019;68:159–171. - PubMed
-
- Caisová V, Uher O, Nedbalová P, Jochmanová I, Kvardová K, Masáková K, Krejčová G, Pad’ouková L, Chmelař J, Kopecký J, et al. Effective cancer immunotherapy based on combination of TLR agonists with stimulation of phagocytosis. Int Immunopharmacol. 2018;59:86–96. - PubMed
-
- Uher O, Caisova V, Padoukova L, Kvardova K, Masakova K, Lencova R, Frejlachova A, Skalickova M, Venhauerova A, Chlastakova A, et al. Mannan-BAM, TLR ligands, and anti-CD40 immunotherapy in established murine pancreatic adenocarcinoma: Understanding therapeutic potentials and limitations. Cancer Immunol Immun. 2021; doi: 10.1007/s00262-021-02920-9. - DOI - PMC - PubMed
-
- Janotová T, Jalovecká M, Auerová M, Švecová I, Bruzlová P, Maierová V, Kumžáková Z, Čunátová Š, Vlčková Z, Caisová V, et al. The use of anchored agonists of phagocytic receptors for cancer immunotherapy: B16-F10 murine melanoma model. PLoS ONE. 2014;9: e85222. doi:10.1371/journal.pone.0085222. - DOI - PMC - PubMed
-
- Waldmannová E, Caisová V, Fáberová J, Sváčková P, Kovářová M, Sváčková D, Kumžáková Z, Jačková A, Vácová N, Nedbalová P, et al. The use of Zymosan A and bacteria anchored to tumor cells for effective cancer immunotherapy: B16-F10 murine melanoma model. Int Immunopharmacol. 2016;39:295–306. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

