A role for protein arginine methyltransferase 7 in repetitive and mild traumatic brain injury
- PMID: 37030326
- PMCID: PMC10988608
- DOI: 10.1016/j.neuint.2023.105524
A role for protein arginine methyltransferase 7 in repetitive and mild traumatic brain injury
Abstract
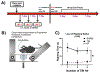
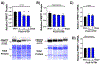
Mild traumatic brain injury affects the largest proportion of individuals in the United States and world-wide. Pre-clinical studies of repetitive and mild traumatic brain injury (rmTBI) have been limited in their ability to recapitulate human pathology (i.e. diffuse rotational injury). We used the closed-head impact model of engineered rotation acceleration (CHIMERA) to simulate rotational injury observed in patients and to study the pathological outcomes post-rmTBI using C57BL/6J mice. Enhanced cytokine production was observed in both the cortex and hippocampus to suggest neuroinflammation. Furthermore, microglia were assessed via enhanced iba1 protein levels and morphological changes using immunofluorescence. In addition, LC/MS analyses revealed excess glutamate production, as well as diffuse axonal injury via Bielschowsky's silver stain kit. Moreover, the heterogeneous nature of rmTBI has made it challenging to identify drug therapies that address rmTBI, therefore we sought to identify novel targets in the concurrent rmTBI pathology. The pathophysiological findings correlated with a time-dependent decrease in protein arginine methyltransferase 7 (PRMT7) protein expression and activity post-rmTBI along with dysregulation of PRMT upstream mediators s-adenosylmethionine and methionine adenosyltransferase 2 (MAT2) in vivo. In addition, inhibition of the upstream mediator MAT2A using the HT22 hippocampal neuronal cell line suggest a mechanistic role for PRMT7 via MAT2A in vitro. Collectively, we have identified PRMT7 as a novel target in rmTBI pathology in vivo and a mechanistic link between PRMT7 and upstream mediator MAT2A in vitro.
Keywords: Methionine adenosyltransferase 2; PF-9366; Protein arginine methyltransferase; S-adenosylmethionine; Traumatic brain injury.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest.
Figures

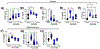





References
-
- Cali E, Suri M, Scala M, Ferla MP, Alavi S, Faqeih EA, Maroofian R, 2022. Biallelic PRMT7 pathogenic variants are associated with a recognizable syndromic neurodevelopmental disorder with short stature, obesity, and craniofacial and digital abnormalities. Genet. Med 10.1016/j.gim.2022.09.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

