Fibroblast growth factor-21 is required for weight loss induced by the glucagon-like peptide-1 receptor agonist liraglutide in male mice fed high carbohydrate diets
- PMID: 37030441
- PMCID: PMC10131131
- DOI: 10.1016/j.molmet.2023.101718
Fibroblast growth factor-21 is required for weight loss induced by the glucagon-like peptide-1 receptor agonist liraglutide in male mice fed high carbohydrate diets
Abstract
Objective: Glucagon-like peptide-1 receptor (GLP-1R) agonists (GLP-1RA) and fibroblast growth factor-21 (FGF21) confer similar metabolic benefits. GLP-1RA induce FGF21, leading us to investigate mechanisms engaged by the GLP-1RA liraglutide to increase FGF21 levels and the metabolic relevance of liraglutide-induced FGF21.
Methods: Circulating FGF21 levels were measured in fasted male C57BL/6J, neuronal GLP-1R knockout, β-cell GLP-1R knockout, and liver peroxisome proliferator-activated receptor alpha knockout mice treated acutely with liraglutide. To test the metabolic relevance of liver FGF21 in response to liraglutide, chow-fed control and liver Fgf21 knockout (LivFgf21-/-) mice were treated with vehicle or liraglutide in metabolic chambers. Body weight and composition, food intake, and energy expenditure were measured. Since FGF21 reduces carbohydrate intake, we measured body weight in mice fed matched diets with low- (LC) or high-carbohydrate (HC) content and in mice fed a high-fat, high-sugar (HFHS) diet. This was done in control and LivFgf21-/- mice and in mice lacking neuronal β-klotho (Klb) expression to disrupt brain FGF21 signaling.
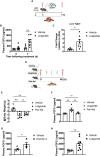
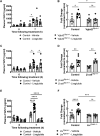
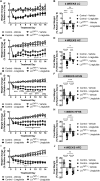
Results: Liraglutide increases FGF21 levels independently of decreased food intake via neuronal GLP-1R activation. Lack of liver Fgf21 expression confers resistance to liraglutide-induced weight loss due to attenuated reduction of food intake in chow-fed mice. Liraglutide-induced weight loss was impaired in LivFgf21-/- mice when fed HC and HFHS diets but not when fed a LC diet. Loss of neuronal Klb also attenuated liraglutide-induced weight loss in mice fed HC or HFHS diets.
Conclusions: Our findings support a novel role for a GLP-1R-FGF21 axis in regulating body weight in a dietary carbohydrate-dependent manner.
Keywords: Carbohydrate; Fibroblast growth factor-21; Food intake; Glucagon-like peptide-1 receptor agonists; Liraglutide; Weight loss.
Copyright © 2023 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


Update of
-
Liver Fibroblast Growth Factor 21 (FGF21) is Required for the Full Anorectic Effect of the Glucagon-Like Peptide-1 Receptor Agonist Liraglutide in Male Mice fed High Carbohydrate Diets.bioRxiv [Preprint]. 2023 Jan 3:2023.01.03.522509. doi: 10.1101/2023.01.03.522509. bioRxiv. 2023. Update in: Mol Metab. 2023 Jun;72:101718. doi: 10.1016/j.molmet.2023.101718. PMID: 36711605 Free PMC article. Updated. Preprint.
References
-
- Fryar C.D., Carroll M.D., Afful J. Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats. 2020
-
- Stierman B., Afful J., Carroll M.D., Chen T.C., Davy O., Fink S., et al. National Health and Nutrition Examination Survey 2017–March 2020 prepandemic data files development of files and prevalence estimates for selected health outcomes. National Health Stat Rep. 2021;2021(158) doi: 10.15620/CDC:106273. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- U2C DK135073/DK/NIDDK NIH HHS/United States
- R01 DK125353/DK/NIDDK NIH HHS/United States
- U2C DK059637/DK/NIDDK NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
- R01 DK123075/DK/NIDDK NIH HHS/United States
- R01 DK106104/DK/NIDDK NIH HHS/United States
- T32 DK007563/DK/NIDDK NIH HHS/United States
- R01 DK046492/DK/NIDDK NIH HHS/United States
- S10 OD028455/OD/NIH HHS/United States
- S10 OD021680/OD/NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- IK2 BX005715/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

