Translational and Posttranslational Dynamics in a Model Peptidergic System
- PMID: 37030596
- PMCID: PMC10205546
- DOI: 10.1016/j.mcpro.2023.100544
Translational and Posttranslational Dynamics in a Model Peptidergic System
Abstract
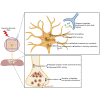
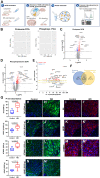
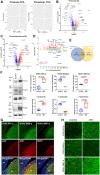
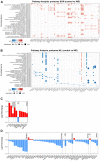
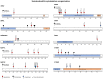
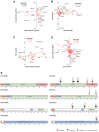
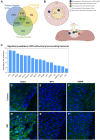
The cell bodies of hypothalamic magnocellular neurones are densely packed in the hypothalamic supraoptic nucleus, whereas their axons project to the anatomically discrete posterior pituitary gland. We have taken advantage of this unique anatomical structure to establish proteome and phosphoproteome dynamics in neuronal cell bodies and axonal terminals in response to physiological stimulation. We have found that proteome and phosphoproteome responses to neuronal stimulation are very different between somatic and axonal neuronal compartments, indicating the need of each cell domain to differentially adapt. In particular, changes in the phosphoproteome in the cell body are involved in the reorganization of the cytoskeleton and in axonal terminals the regulation of synaptic and secretory processes. We have identified that prohormone precursors including vasopressin and oxytocin are phosphorylated in axonal terminals and are hyperphosphorylated following stimulation. By multiomic integration of transcriptome and proteomic data, we identify changes to proteins present in afferent inputs to this nucleus.
Keywords: Axonal terminal; Cell body; Cytoskeleton; Magnocellular neurones; Phosphoproteome; Proteome; Synapse.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest.
Figures


References
-
- Burbach J.P., Luckman S.M., Murphy D., Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol. Rev. 2001;81:1197–1267. - PubMed
-
- Mecawi A.S., Ruginsk S.G., Elias L.L., Varanda W.A., Antunes-Rodrigues J. Neuroendocrine regulation of hydromineral homeostasis. Compr. Physiol. 2015;5:1465–1516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

