Side effects of COVID-19 vaccinations in patients treated for breast cancer
- PMID: 37031282
- PMCID: PMC10098240
- DOI: 10.1007/s10238-023-01050-z
Side effects of COVID-19 vaccinations in patients treated for breast cancer
Abstract
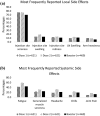
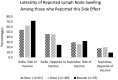
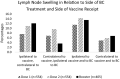
Lymph node swelling is a side effect of the mRNA COVID-19 vaccines, a distressing side effect for women treated for breast cancer. The purpose of this study is to present side effects reported by a cohort of patients treated for breast cancer. A survey link was sent to 4945 women who received breast cancer treatment and were prospectively screened for breast cancer-related lymphedema. In total, 621 patients who received an mRNA vaccine and responded to the survey were included in analysis. We assessed the frequency and predictors of side effects. The most frequent side effects reported were injection site soreness, fatigue, generalized muscle soreness, headache, and chills, with median duration ≤ 48 h. Lymph node swelling occurred most often in the axilla ipsilateral to the vaccine. The median duration was 1 week or less after all doses. These data will inform patient education regarding future vaccine doses, including reassurances about which side effects to expect, particularly lymph node swelling which may impact mammograms after vaccination. Type and duration of side effects were similar to that reported by the general population in Phase 3 testing trials of the mRNA vaccines. Clinical Trial Registration NCT04872738 posted May 4, 2021.
Trial registration: ClinicalTrials.gov NCT048727384 NCT04872738 NCT48727384.
Keywords: Breast cancer; COVID-19; Lymphedema; Lymphoedema; Vaccines.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Alphonse G. Taghian has been loaned equipment from ImpediMed for use in an investigator-initiated clinical trial. Alphonse G. Taghian is on the Scientific Advisory Board of Puretech Health (not paid) and is a previous consultant for VisionRT. Both involvements are unrelated to this study. Cheryl L. Brunelle is on the Scientific Advisory Board of Puretech Health. The remaining authors have no conflict of interest.
Figures
References
-
- Recurrent Breast Cancer. Mayo Clin. 2022; Available from: https://www.mayoclinic.org/diseases-conditions/recurrent-breast-cancer/s...
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

