A tailored tetravalent peptide displays dual functions to inhibit amyloid β production and aggregation
- PMID: 37031306
- PMCID: PMC10082830
- DOI: 10.1038/s42003-023-04771-9
A tailored tetravalent peptide displays dual functions to inhibit amyloid β production and aggregation
Abstract
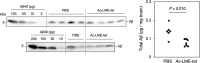
Inhibition of amyloid-β peptide (Aβ) accumulation in the brain is a promising approach for treatment of Alzheimer's disease (AD). Aβ is produced by β-secretase and γ-secretase in endosomes via sequential proteolysis of amyloid precursor protein (APP). Aβ and APP have a common feature to readily cluster to form multimers. Here, using multivalent peptide library screens, we identified a tetravalent peptide, LME-tet, which binds APP and Aβ via multivalent interactions. In cells, LME-tet-bound APP in the plasma membrane is transported to endosomes, blocking Aβ production through specific inhibition of β-cleavage, but not γ-cleavage. LME-tet further suppresses Aβ aggregation by blocking formation of the β-sheet conformation. Inhibitory effects are not observed with a monomeric peptide, emphasizing the significance of multivalent interactions for mediating these activities. Critically, LME-tet efficiently reduces Aβ levels in the brain of AD model mice, suggesting it may hold promise for treatment of AD.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

