High baseline tumor burden-associated macrophages promote an immunosuppressive microenvironment and reduce the efficacy of immune checkpoint inhibitors through the IGFBP2-STAT3-PD-L1 pathway
- PMID: 37031362
- PMCID: PMC10174084
- DOI: 10.1002/cac2.12420
High baseline tumor burden-associated macrophages promote an immunosuppressive microenvironment and reduce the efficacy of immune checkpoint inhibitors through the IGFBP2-STAT3-PD-L1 pathway
Abstract
Background: Several clinical studies have uncovered a negative correlation between baseline tumor burden and the efficacy of immune checkpoint inhibitor (ICI) treatment. This study aimed to uncover the specific mechanisms underlying the difference in sensitivity to ICI treatment between tumors with high (HTB) and low (LTB) tumor burden.
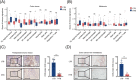
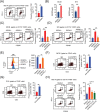
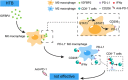
Methods: For in vivo studies, several mouse models of subcutaneous tumors were established, and transcriptome sequencing, immunohistochemistry, and flow cytometry assays were used to detect the immune status in these subcutaneous tumors. For in vitro experiments, co-culture models, cytokine antibody arrays, western blotting, flow cytometry, and enzyme-linked immunosorbent assays were used to explore the underlying molecular mechanisms RESULTS: We found that MC38 or B16 subcutaneous tumors from the HTB group did not show any response to anti-programmed cell death protein-1 (PD-1) therapy. Through flow cytometry assays, we found that the infiltration with CD8+ T cells was significantly decreased whereas M2-like macrophages were enriched in subcutaneous tumors of HTB groups compared with those of LTB group. These changes were not affected by the initial number of injected tumor cells or tumor age, nor could they be reversed by surgical tumor reduction. Intraperitoneal colony-stimulating factor 1 receptor (CSF-1R) inhibitor PLX3397 injection at different time points of tumor growth only had an effect when administered in the early tumor stage to maintain the "heat" of the tumor microenvironment during the process of tumor growth, thereby achieving a response to ICI treatment when the tumor grew to a large size. Mechanistically, we found that insulin-like growth factor binding protein 2 (IGFBP2) expression levels were significantly elevated in HTB tumor tissues. IGFBP2 promoted the programmed death-ligand 1 (PD-L1) expression in M2-like macrophages by activating signal transducer and activator of transcription 3 (STAT3), and PD-L1+ M2-like macrophages exerted an immunosuppressive effect by inhibiting the proliferation and activation of CD8+ T cells in a PD-L1-dependent fashion.
Conclusions: This study suggested that the low efficacy of ICI treatment in HTB tumors is mainly attributed to the intratumoral accumulation of PD-L1+ M2-like macrophages via the IGFBP2-STAT3-PD-L1 signaling pathway and their substantial inhibitory effects on T cell proliferation and activation.
Keywords: CD8+ T cell; IGFBP2; PD-L1; STAT3; immune checkpoint inhibitor; macrophage; tumor burden; tumor immune microenvironment.
© 2023 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Hopkins AM, Kichenadasse G, McKinnon RA, Rowland A, Sorich MJ. Baseline tumor size and survival outcomes in lung cancer patients treated with immune checkpoint inhibitors. Semin Oncol. 2019;46(4‐5):380–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

