Plasma p-tau181 and p-tau217 in discriminating PART, AD and other key neuropathologies in older adults
- PMID: 37031430
- PMCID: PMC10261204
- DOI: 10.1007/s00401-023-02570-4
Plasma p-tau181 and p-tau217 in discriminating PART, AD and other key neuropathologies in older adults
Abstract
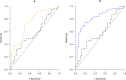
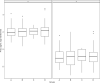
We examined whether plasma p-tau181 and p-tau217 are specific biomarkers of pathologically confirmed Alzheimer's disease (AD). In particular, we investigated the utility of plasma p-tau for differentiating AD from primary age-related tauopathy (PART), as well as AD with mixed pathologies. Data came from 269 older adults who participated in the Religious Orders Study or the Rush Memory and Aging Project. Blood samples were collected during annual clinical evaluations. Participants died and underwent brain autopsy. P-tau181 and p-tau217 were quantified in the plasma samples proximate to death (average interval before death: 1.4 years) using Lilly-developed MSD immunoassays. Uniform neuropathologic evaluations assessed AD, PART, and other common degenerative and cerebrovascular conditions. Plasma p-tau217 was more strongly correlated with brain β-amyloid and paired helical filament tau (PHFtau) tangles than p-tau181. Both p-tau markers were associated with greater odds of AD, but p-tau217 had higher accuracy (area under the ROC curve (AUC): 0.83) than p-tau181 (AUC: 0.76). Plasma p-tau markers were almost exclusively associated with AD pathologic indices with the exception of cerebral amyloid angiopathy. Compared to p-tau181, p-tau217 showed a higher AUC (0.82 versus 0.74) in differentiating AD from PART. For either p-tau, we did not observe a level difference between individuals with AD alone and those with mixed AD pathologies. In summary, plasma p-tau181and p-tau217 were specifically associated with AD pathological changes. Further, our data provide initial evidence that p-tau217 may be able to differentiate between AD and PART in individuals with comparable burdens of tau tangle pathology. These results demonstrate the specificity of p-tau217 for AD, supporting its use to identify patients suitable for anti-AD therapies including β-amyloid immunotherapies.
Keywords: Alzheimer’s disease; Mixed pathologies; PART; Plasma p-tau217; p-tau181.
© 2023. The Author(s).
Conflict of interest statement
OH has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Genentech, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. JAS receives consulting fee from Alnylam Pharmaceuticals and Cerveau Technologies. The other authors declare no competing interests or conflicts of interest.
Figures

References
-
- Agrawal S, Yu L, Kapasi A, James BD, Arfanakis K, Barnes LL, Bennett DA, Nag S, Schneider JA. Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change and microvascular pathologies in community-dwelling older persons. Brain Pathol. 2021;31:e12939–e12939. doi: 10.1111/bpa.12939. - DOI - PMC - PubMed
-
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

