A systematic review on the performance of fracture risk assessment tools: FRAX, DeFRA, FRA-HS
- PMID: 37031450
- PMCID: PMC10558377
- DOI: 10.1007/s40618-023-02082-8
A systematic review on the performance of fracture risk assessment tools: FRAX, DeFRA, FRA-HS
Abstract
Purpose: Preventing fragility fractures by treating osteoporosis may reduce disability and mortality worldwide. Algorithms combining clinical risk factors with bone mineral density have been developed to better estimate fracture risk and possible treatment thresholds. This systematic review supported panel members of the Italian Fragility Fracture Guidelines in recommending the use of best-performant tool. The clinical performance of the three most used fracture risk assessment tools (DeFRA, FRAX, and FRA-HS) was assessed in at-risk patients.
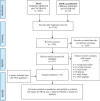
Methods: PubMed, Embase, and Cochrane Library were searched till December 2020 for studies investigating risk assessment tools for predicting major osteoporotic or hip fractures in patients with osteoporosis or fragility fractures. Sensitivity (Sn), specificity (Sp), and areas under the curve (AUCs) were evaluated for all tools at different thresholds. Quality assessment was performed using the Quality Assessment of Diagnostic Accuracy Studies-2; certainty of evidence (CoE) was evaluated using the Grading of Recommendations Assessment, Development and Evaluation approach.
Results: Forty-three articles were considered (40, 1, and 2 for FRAX, FRA-HS, and DeFRA, respectively), with the CoE ranging from very low to high quality. A reduction of Sn and increase of Sp for major osteoporotic fractures were observed among women and the entire population with cut-off augmentation. No significant differences were found on comparing FRAX to DeFRA in women (AUC 59-88% vs. 74%) and diabetics (AUC 73% vs. 89%). FRAX demonstrated non-significantly better discriminatory power than FRA-HS among men.
Conclusion: The task force formulated appropriate recommendations on the use of any fracture risk assessment tools in patients with or at risk of fragility fractures, since no statistically significant differences emerged across different prediction tools.
Keywords: Fracture risk assessment; Fragility fracture; Secondary prevention; Systematic review.
© 2023. The Author(s).
Conflict of interest statement
GA declares personal fees from Theramex, Amgen, BMS, Lilly, Fresenius Kabi and Galapagos. LC declares personal fees from UCB Pharma, Abiogen Pharma, Bruno Farmaceutici, Sandoz, Metagenics. DG has received honoraria as consultant for Eli-Lilly, Organon, MSD Italia. SG has received honoraria as consultant for UCB Pharma. SM has received honoraria as consultant for UCB, Eli-Lilly, Amgen. MLB has received (i) honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB, (ii) grants and/or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, UCB Pharma, (iii) consultant: Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB Pharma. GC received research support from the European Community (EC), the Italian Agency of Drug (AIFA), and the Italian Ministry for University and Research (MIUR). He took part to a variety of projects that were funded by pharmaceutical companies (i.e., Novartis, GSK, Roche, AMGEN and BMS). He also received honoraria as member of Advisory Board from Roche. No other potential conflicts of interest relevant to this article were disclosed. MR declares personal fees from Amgen, ABBvie, BMS, Eli Lilly, Galapagos, Menarini, Novartis, Pfizer, Sandoz, Theramex and UCB outside the submitted work. RM took part to a project funded by Abiogen Pharma. GI received honoraria as speaker by Eli-Lilly, Menarini, UCB Pharma. The other authors declare that they have no conflict of interest. All authors have completed the ICMJE uniform disclosure form at
References
-
- Adami S, Bianchi G, Brandi ML, Di Munno O, Frediani B, Gatti D, et al. Validation and further development of the WHO 10-year fracture risk assessment tool in Italian postmenopausal women: project rationale and description. Clin Exp Rheumatol. 2010;28:561–570. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical