Synthesis and anti-SARS-CoV-2 evaluation of lipid prodrugs of β-D-N4-hydroxycytidine (NHC) and a 3'-fluoro-substituted analogue of NHC
- PMID: 37031504
- PMCID: PMC10076076
- DOI: 10.1016/j.bioorg.2023.106527
Synthesis and anti-SARS-CoV-2 evaluation of lipid prodrugs of β-D-N4-hydroxycytidine (NHC) and a 3'-fluoro-substituted analogue of NHC
Abstract
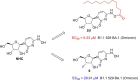
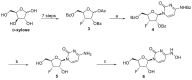
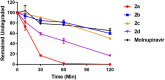
β-D-N4-hydroxycytidine (NHC, EIDD-1931) is a nucleoside analogue that exhibits broad spectrum antiviral activity against a variety of RNA viruses. Herein, we report the synthesis of a series of lipid prodrugs of NHC and a novel 3'-fluoro modified NHC analogue, and evaluation of their antiviral activity against five variants of SARS-CoV-2. All lipid prodrugs showed potent antiviral activity against the tested SARS-CoV-2 variants with EC50 values in the range of 0.31-3.51 μM, which were comparable to those of NHC or higher than those of remdesivir and molnupiravir. An increase in the cytostatic activity of the lipid prodrugs was found, but prodrug 2d proved equally selective as molnupinavir. The 3'-F analogue of NHC (6) only displayed minor antiviral activity against the SARS-CoV-2 Omicron variant (EC50 = 29.91 μM), while no activity was found for other variants at the highest concentration tested. The promising antiviral data of the lipid prodrugs of NHC suggest that they deserve further investigation as new anti-SARS-CoV-2 drugs.
Keywords: COVID-19; EIDD-1931; Lipid prodrugs; Nucleoside analogue; SARS-CoV-2.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
New conjugates based on N4-hydroxycytidine with more potent antiviral efficacy in vitro than EIDD-2801 against SARS-CoV-2 and other human coronaviruses.Antiviral Res. 2024 May;225:105871. doi: 10.1016/j.antiviral.2024.105871. Epub 2024 Mar 28. Antiviral Res. 2024. PMID: 38555022
-
Transfer and biotransformation of the COVID-19 prodrug molnupiravir and its metabolite β-D-N4-hydroxycytidine across the blood-placenta barrier.EBioMedicine. 2023 Sep;95:104748. doi: 10.1016/j.ebiom.2023.104748. Epub 2023 Aug 4. EBioMedicine. 2023. PMID: 37544201 Free PMC article.
-
Molnupiravir maintains antiviral activity against SARS-CoV-2 variants and exhibits a high barrier to the development of resistance.Antimicrob Agents Chemother. 2024 Jan 10;68(1):e0095323. doi: 10.1128/aac.00953-23. Epub 2023 Dec 4. Antimicrob Agents Chemother. 2024. PMID: 38047645 Free PMC article.
-
Molnupiravir and Its Antiviral Activity Against COVID-19.Front Immunol. 2022 Apr 4;13:855496. doi: 10.3389/fimmu.2022.855496. eCollection 2022. Front Immunol. 2022. PMID: 35444647 Free PMC article. Review.
-
Broad-spectrum prodrugs with anti-SARS-CoV-2 activities: Strategies, benefits, and challenges.J Med Virol. 2022 Apr;94(4):1373-1390. doi: 10.1002/jmv.27517. Epub 2021 Dec 18. J Med Virol. 2022. PMID: 34897729 Review.
Cited by
-
Effective, but Safe? Physiologically Based Pharmacokinetic (PBPK)-Modeling-Based Dosing Study of Molnupiravir for Risk Assessment in Pediatric Subpopulations.ACS Pharmacol Transl Sci. 2024 Nov 27;7(12):4112-4122. doi: 10.1021/acsptsci.4c00535. eCollection 2024 Dec 13. ACS Pharmacol Transl Sci. 2024. PMID: 39698289
-
Synthesis and Antiviral Activity of Novel β-D-N4-Hydroxycytidine Ester Prodrugs as Potential Compounds for the Treatment of SARS-CoV-2 and Other Human Coronaviruses.Pharmaceuticals (Basel). 2023 Dec 26;17(1):35. doi: 10.3390/ph17010035. Pharmaceuticals (Basel). 2023. PMID: 38256869 Free PMC article.
References
-
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. - PubMed
-
- Gil C., Ginex T., Maestro I., Nozal V., Barrado-Gil L., Cuesta-Geijo M.A., Urquiza J., Ramirez D., Alonso C., Campillo N.E., Martinez A. COVID-19: drug targets and potential treatments. J. Med. Chem. 2020;63(21):12359–12386. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

