Anakinra for Refractory Cytokine Release Syndrome or Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T Cell Therapy
- PMID: 37031746
- PMCID: PMC10330552
- DOI: 10.1016/j.jtct.2023.04.001
Anakinra for Refractory Cytokine Release Syndrome or Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T Cell Therapy
Abstract
Chimeric antigen receptor-engineered (CAR)-T cell therapy remains limited by significant toxicities, including cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). The optimal management of severe and/or refractory CRS/ICANS remains ill-defined. Anakinra has emerged as a promising agent based on preclinical data, but its safety and efficacy in CAR-T therapy recipients are unknown. The primary objective of this study was to evaluate the safety of anakinra to treat refractory CRS and ICANS after CAR-T therapy. The secondary objective was to evaluate the impact of key treatment-, patient-, and disease-related variables on the time to CRS/ICANS resolution and treatment-related mortality (TRM). We retrospectively analyzed the outcomes of 43 patients with B cell or plasma cell malignancies treated with anakinra for refractory CRS or ICANS at 9 institutions in the United States and Spain between 2019 and 2022. Cause-specific Cox regression was used to account for competing risks. Multivariable cause-specific Cox regression was used to estimate the effect of anakinra dose on outcomes while minimizing treatment allocation bias by including age, CAR-T product, prelymphodepletion (pre-LD) ferritin, and performance status. Indications for anakinra treatment were grade ≥2 ICANS with worsening or lack of symptom improvement despite treatment with high-dose corticosteroids (n = 40) and grade ≥2 CRS with worsening symptoms despite treatment with tocilizumab (n = 3). Anakinra treatment was feasible and safe; discontinuation of therapy because of anakinra-related side effects was reported in only 3 patients (7%). The overall response rate (ORR) to CAR-T therapy was 77%. The cumulative incidence of TRM in the whole cohort was 7% (95% confidence interval [CI], 2% to 17%) at 28 days and 23% (95% CI, 11% to 38%) at 60 days after CAR-T infusion. The cumulative incidence of TRM at day 28 after initiation of anakinra therapy was 0% in the high-dose (>200 mg/day i.v.) recipient group and 47% (95% CI, 20% to 70%) in the low-dose (100 to 200 mg/day s.c. or i.v.) recipient group. The median cumulative incidence of CRS/ICANS resolution from the time of anakinra initiation was 7 days in the high-dose group and was not reached in the low-dose group, owing to the high TRM in this group. Univariate Cox modeling suggested a shorter time to CRS/ICANS resolution in the high-dose recipients (hazard ratio [HR], 2.19; 95% CI, .94 to 5.12; P = .069). In a multivariable Cox model for TRM including age, CAR-T product, pre-LD ferritin level, and pre-LD Karnofsky Performance Status (KPS), higher anakinra dose remained associated with lower TRM (HR, .41 per 1 mg/kg/day increase; 95% CI, .17 to .96; P = .039. The sole factor independently associated with time to CRS/ICANS resolution in a multivariable Cox model including age, CAR-T product, pre-LD ferritin and anakinra dose was higher pre-LD KPS (HR, 1.05 per 10% increase; 95% CI, 1.01 to 1.09; P = .02). Anakinra treatment for refractory CRS or ICANS was safe at doses up to 12 mg/kg/day i.v. We observed an ORR of 77% after CAR-T therapy despite anakinra treatment, suggesting a limited impact of anakinra on CAR-T efficacy. Higher anakinra dose may be associated with faster CRS/ICANS resolution and was independently associated with lower TRM. Prospective comparative studies are needed to confirm our findings.
Keywords: Anakinra; CAR-T cell therapy; CRS; ICANS; Toxicity.
Published by Elsevier Inc.
Conflict of interest statement
Conflicts of interest
Figures

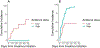
Comment in
-
Thinking Clearly with Anakinra.Transplant Cell Ther. 2023 Jul;29(7):406-407. doi: 10.1016/j.jtct.2023.06.004. Transplant Cell Ther. 2023. PMID: 37400190 No abstract available.
References
-
- Research C for DE and. FDA approves brexucabtagene autoleucel for relapsed or refractory B-cell precursor acute lymphoblastic leukemia. FDA. Published online January 31, 2022. Accessed May 31, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

