Comorbidities and the use of comedications among patients with chronic hepatitis C in Korea: A nationwide cross-sectional study
- PMID: 37032119
- PMCID: PMC10175864
- DOI: 10.3904/kjim.2022.215
Comorbidities and the use of comedications among patients with chronic hepatitis C in Korea: A nationwide cross-sectional study
Abstract
Background/aims: Chronic hepatitis C (CHC) is the second leading cause of liver-related mortality and is more prevalent in the elderly population in Korea. Decisions to initiate treatment and selection of proper antiviral agents may be challenging among elderly patients due to relevant comorbidities, comedications, and drug-drug interaction (DDI). It may be helpful to understand the current demographic status and comorbidities of CHC patients in the country.
Methods: Patients aged ≥ 18 years and diagnosed with CHC (KCD-7 code B18.2) were extracted from the Korean Health Insurance Review & Assessment Service database in 2018. Data on comorbidities and comedications were assessed and potential DDIs were analyzed.
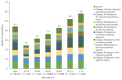
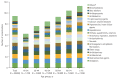
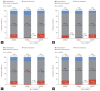
Results: A total of 50,476 patients with CHC, with a mean age of 60.3 years and 46.7% male patients were identified. The proportion of patients with cirrhosis, hepatocellular carcinoma, and liver transplantation was 6.0%, 4.1%, and 0.3%, respectively and 37.2% of patients were more than 65 years of age. The three most common comorbidities were diseases of the digestive system (83.7%), respiratory system (58.2%), and musculoskeletal system and connective tissue (57.6%). The three most common comedications were analgesics (91.6%), gastrointestinal agents (85%), and antibacterials (80.3%). Lipid-lowering agents and anticonvulsants were prescribed in 28.5% and 14.8% of patients. Rate of potential DDI for contraindication was 2.2%, 13.1%, and 15.6% with sofosbuvir/velpatasvir, ledipasvir/sofosbuvir, and glecaprevir/pibrentasvir.
Conclusion: With the increasing age of patients with CHC, comorbidity, comedication, and potential DDI should be considered when choosing antivirals in Korea. Sofosbuvir-based regimens showed favorable DDI profiles among Korean patients.
Keywords: Antiviral agents/therapeutic use; Comorbidity; Drug interactions; Hepatitis C; Polypharmacy.
Conflict of interest statement
Kyung Min Kwon is an employee of Gilead Sciences. Jae-Jun Shim is an employee of the Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea, and Cheorwon Hospital, Gangwon-do, Korea. Gi-Ae Kim is an employee of the Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea. Bo Ok Kim, Helin Han, and Hyun Jung Ahn are employees of Cerner Enviza.
Figures

References
-
- Wei L, Kamae I, Lee MH, Li H. Hepatitis C viral infection disease burden in Asia. Value Outcomes Spotlight. 2015;1:16–18.
-
- The Free Dictionary . Korean Association for the Study of the Liver [Internet] Pennsylvania (GA): AcronymFinder.com; 2020. [cited 2023 Jan 8]. Available from: https://acronyms.thefreedictionary.com/Korean+Association+for+the+-Study... .
-
- Kondili LA, Craxì A, Aghemo A. Absolute targets for HCV elimination and national health policy paradigms: foreseeing future requirements. Liver Int. 2021;41:649–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
