Source attribution of campylobacteriosis in Australia, 2017-2019
- PMID: 37032319
- PMCID: PMC10947381
- DOI: 10.1111/risa.14138
Source attribution of campylobacteriosis in Australia, 2017-2019
Abstract
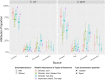
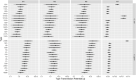
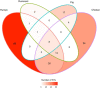
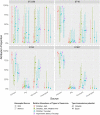
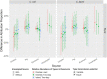
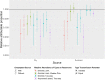
Campylobacter jejuni and Campylobacter coli infections are the leading cause of foodborne gastroenteritis in high-income countries. Campylobacter colonizes a variety of warm-blooded hosts that are reservoirs for human campylobacteriosis. The proportions of Australian cases attributable to different animal reservoirs are unknown but can be estimated by comparing the frequency of different sequence types in cases and reservoirs. Campylobacter isolates were obtained from notified human cases and raw meat and offal from the major livestock in Australia between 2017 and 2019. Isolates were typed using multi-locus sequence genotyping. We used Bayesian source attribution models including the asymmetric island model, the modified Hald model, and their generalizations. Some models included an "unsampled" source to estimate the proportion of cases attributable to wild, feral, or domestic animal reservoirs not sampled in our study. Model fits were compared using the Watanabe-Akaike information criterion. We included 612 food and 710 human case isolates. The best fitting models attributed >80% of Campylobacter cases to chickens, with a greater proportion of C. coli (>84%) than C. jejuni (>77%). The best fitting model that included an unsampled source attributed 14% (95% credible interval [CrI]: 0.3%-32%) to the unsampled source and only 2% to ruminants (95% CrI: 0.3%-12%) and 2% to pigs (95% CrI: 0.2%-11%) The best fitting model that did not include an unsampled source attributed 12% to ruminants (95% CrI: 1.3%-33%) and 6% to pigs (95% CrI: 1.1%-19%). Chickens were the leading source of human Campylobacter infections in Australia in 2017-2019 and should remain the focus of interventions to reduce burden.
Keywords: Bayesian analysis; Campylobacter; source attribution.
© 2023 The Authors. Risk Analysis published by Wiley Periodicals LLC on behalf of Society for Risk Analysis.
Figures

References
-
- Australian Bureau of Agricultural and Resource Economics and Sciences . (2020). Agricultural commodity statistics. https://www.agriculture.gov.au/abares/research‐topics/agricultural‐outlo...
-
- Bolton, D. J. (2015). Campylobacter virulence and survival factors. Food Microbiology, 48, 99–108. - PubMed
-
- Cribb, D. M. , Varrone, L. , Wallace, R. L. , Mclure, A. T. , Smith, J. J. , Stafford, R. J. , Bulach, D. M. , Selvey, L. A. , Firestone, S. M. , French, N. P. , Valcanis, M. , Fearnley, E. J. , Sloan‐Gardner, T. , Graham, T. , Glass, K. , & Kirk, M. D. (2022). Risk factors for campylobacteriosis in Australia: Outcomes of a 2018–2019 case‐control study. 10.21203/rs.3.rs-1387051/v1 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

