Role of neoadjuvant chemoradiotherapy in liver transplantation for unresectable perihilar cholangiocarcinoma: multicentre, retrospective cohort study
- PMID: 37032423
- PMCID: PMC10083139
- DOI: 10.1093/bjsopen/zrad025
Role of neoadjuvant chemoradiotherapy in liver transplantation for unresectable perihilar cholangiocarcinoma: multicentre, retrospective cohort study
Abstract
Background: The Mayo protocol for liver transplantation in patients with unresectable perihilar cholangiocarcinoma is based on strict selection and neoadjuvant chemoradiotherapy. The role of neoadjuvant chemoradiotherapy in this scenario remains unclear. The aim of this study was to compare outcomes after transplantation for perihilar cholangiocarcinoma using strict selection criteria, either with or without neoadjuvant chemoradiotherapy.
Methods: This was an international, multicentre, retrospective cohort study of patients who underwent transplantation between 2011 and 2020 for unresectable perihilar cholangiocarcinoma using the Mayo selection criteria and receiving neoadjuvant chemoradiotherapy or not receiving neoadjuvant chemoradiotherapy. Endpoints were post-transplant survival, post-transplant morbidity rate, and time to recurrence.
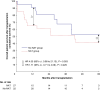
Results: Of 49 patients who underwent liver transplantation for perihilar cholangiocarcinoma, 27 received neoadjuvant chemoradiotherapy and 22 did not. Overall 1-, 3-, and 5-year post-transplantation survival rates were 65 per cent, 51 per cent and 41 per cent respectively in the group receiving neoadjuvant chemoradiotherapy and 91 per cent, 68 per cent and 53 per cent respectively in the group not receiving neoadjuvant chemoradiotherapy (1-year hazards ratio (HR) 4.55 (95 per cent c.i. 0.98 to 21.13), P = 0.053; 3-year HR 2.07 (95 per cent c.i. 0.78 to 5.54), P = 0.146; 5-year HR 1.71 (95 per cent c.i. 0.71 to 4.09), P = 0.229). Hepatic vascular complications were more frequent in the group receiving neoadjuvant chemoradiotherapy compared with the group not receiving neoadjuvant chemoradiotherapy (nine of 27 versus two of 22, P = 0.045). In multivariable analysis, tumour recurrence occurred less frequently in the group receiving neoadjuvant chemoradiotherapy (HR 0.30 (95 per cent c.i. 0.09 to 0.97), P = 0.044).
Conclusion: In selected patients undergoing liver transplantation for perihilar cholangiocarcinoma, neoadjuvant chemoradiotherapy resulted in a lower risk of tumour recurrence, but was associated with a higher rate of early hepatic vascular complications. Adjustments in neoadjuvant chemoradiotherapy reducing the risk of hepatic vascular complications, such as omitting radiotherapy, may further improve the outcome in patients undergoing liver transplantation for perihilar cholangiocarcinoma.
© The Author(s) 2023. Published by Oxford University Press on behalf of BJS Society Ltd.
Figures

Similar articles
-
Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers.Gastroenterology. 2012 Jul;143(1):88-98.e3; quiz e14. doi: 10.1053/j.gastro.2012.04.008. Epub 2012 Apr 12. Gastroenterology. 2012. PMID: 22504095 Free PMC article.
-
Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada.J Surg Oncol. 2018 Feb;117(2):213-219. doi: 10.1002/jso.24833. J Surg Oncol. 2018. PMID: 29480952
-
Single-center experience of liver transplantation for perihilar cholangiocarcinoma.HPB (Oxford). 2022 Apr;24(4):461-469. doi: 10.1016/j.hpb.2021.08.940. Epub 2021 Aug 18. HPB (Oxford). 2022. PMID: 34465528
-
Liver transplantation for cholangiocarcinoma: current best practice.Curr Opin Organ Transplant. 2014 Jun;19(3):245-52. doi: 10.1097/MOT.0000000000000087. Curr Opin Organ Transplant. 2014. PMID: 24811436 Review.
-
Can the Limits of Liver Transplantation Be Expanded in Perihilar Cholangiocarcinoma?J Gastrointest Cancer. 2022 Dec;53(4):1104-1112. doi: 10.1007/s12029-021-00735-6. Epub 2021 Nov 5. J Gastrointest Cancer. 2022. PMID: 34738188 Review.
Cited by
-
Modern therapeutic approaches for hepatic tumors: progress, limitations, and future directions.Discov Oncol. 2025 May 30;16(1):959. doi: 10.1007/s12672-025-02773-z. Discov Oncol. 2025. PMID: 40445470 Free PMC article. Review.
-
Liver Transplantation for Cancer-Current Challenges and Emerging Solutions.J Clin Med. 2025 Jul 29;14(15):5365. doi: 10.3390/jcm14155365. J Clin Med. 2025. PMID: 40806987 Free PMC article. Review.
-
The Impact of Biliary Injury on the Recurrence of Biliary Cancer and Benign Disease after Liver Transplantation: Risk Factors and Mechanisms.Cancers (Basel). 2024 Aug 7;16(16):2789. doi: 10.3390/cancers16162789. Cancers (Basel). 2024. PMID: 39199562 Free PMC article. Review.
-
Neoadjuvant Multiagent Systemic Therapy Approach to Liver Transplantation for Perihilar Cholangiocarcinoma.Transplant Direct. 2025 Feb 7;11(3):e1760. doi: 10.1097/TXD.0000000000001760. eCollection 2025 Mar. Transplant Direct. 2025. PMID: 39936132 Free PMC article.
-
Updates and Expert Opinions on Liver Transplantation for Gastrointestinal Malignancies.Medicina (Kaunas). 2023 Jul 13;59(7):1290. doi: 10.3390/medicina59071290. Medicina (Kaunas). 2023. PMID: 37512101 Free PMC article. Review.
References
-
- Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus Pet al. . New staging system and a registry for perihilar cholangiocarcinoma. Hepatology 2011;53:1363–1371 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

