Comparison of the effects of probiotics, rifaximin, and lactulose in the treatment of minimal hepatic encephalopathy and gut microbiota
- PMID: 37032856
- PMCID: PMC10080009
- DOI: 10.3389/fmicb.2023.1091167
Comparison of the effects of probiotics, rifaximin, and lactulose in the treatment of minimal hepatic encephalopathy and gut microbiota
Abstract
Background: Minimal hepatic encephalopathy (MHE) is an early stage in the pathogenesis of hepatic encephalopathy. Intestinal microbiota is involved in the pathogenesis of hepatic encephalopathy and has become an important therapeutic target. Since there is no unified treatment principle for MHE, this study was conducted to determine the safety and efficacy of different intestinal microecological modulators in the treatment of MHE, and to explore the potential mechanism through intestinal microbiota analysis.
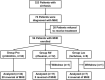
Methods: Patients with liver cirrhosis were screened for MHE using psychometric hepatic encephalopathy score test. Patients diagnosed with MHE were enrolled and received probiotics, rifaximin, or lactulose for 4 weeks. Adverse events were recorded. The psychometric hepatic encephalopathy score test was performed after treatment. Samples of blood and stool were collected at entry and 4 weeks. Blood samples were analyzed to assess blood ammonia, liver, kidney, and hemostatic functions. Stool microbiota were sequenced to confirm changes in microbial composition.
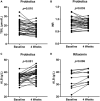
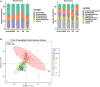
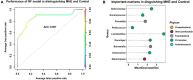
Results: Of 323 patients with liver cirrhosis, 74 patients were diagnosed with MHE. In all, 54 patients were enrolled and 52 who agree to follow-up were included in analysis. The recovery rates of MHE patients received probiotics, rifaximin, and lactulose were 58.8% (20/34), 45.5% (5/11), and 57.1% (4/7), respectively. Probiotics and rifaximin improved liver function in MHE patients to a certain extent. Taxonomic compositions of gut microbiota in MHE patients were distinct from healthy people before treatment; the differences were significantly reduced after treatment, and the gut microbiota gradually resembled the structure of healthy individuals. We found that the relative abundance of specific taxa associated with anti-inflammatory and good cognitive functions was increased in MHE patients after treatment. Accordingly, metabolic pathways in MHE patients were altered before and after treatment. Downregulated pathways after probiotics treatment included glycometabolism and degradation of aromatic compounds. After lactulose treatment, degradation pathways of arginine and ornithine showed a downward trend.
Conclusion: Probiotics, rifaximin, and lactulose are safe and effective in the treatment of MHE, and improve the composition of gut microbiota to some extent.
Keywords: gut microbiota; lactulose; minimal hepatic encephalopathy; probiotics; rifaximin.
Copyright © 2023 Wang, Ma, Wang, Ma, Xue, Guan and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
The effects of rifaximin and lactulose on the gut-liver-brain axis in rats with minimal hepatic encephalopathy.PLoS One. 2025 Jun 17;20(6):e0325988. doi: 10.1371/journal.pone.0325988. eCollection 2025. PLoS One. 2025. PMID: 40526631 Free PMC article.
-
RiMINI - the influence of rifaximin on minimal hepatic encephalopathy (MHE) and on the intestinal microbiome in patients with liver cirrhosis: study protocol for a randomized controlled trial.Trials. 2016 Feb 29;17(1):111. doi: 10.1186/s13063-016-1205-8. Trials. 2016. PMID: 26926775 Free PMC article. Clinical Trial.
-
Long-Term Effect of Rifaximin with and without Lactulose on the Active Bacterial Assemblages in the Proximal Small Bowel and Faeces in Patients with Minimal Hepatic Encephalopathy.Dig Dis. 2019;37(2):161-169. doi: 10.1159/000494216. Epub 2018 Nov 14. Dig Dis. 2019. PMID: 30428474 Clinical Trial.
-
Role of gut microbiota in the pathogenesis and therapeutics of minimal hepatic encephalopathy via the gut-liver-brain axis.World J Gastroenterol. 2023 Jan 7;29(1):144-156. doi: 10.3748/wjg.v29.i1.144. World J Gastroenterol. 2023. PMID: 36683714 Free PMC article. Review.
-
Current approach to treatment of minimal hepatic encephalopathy in patients with liver cirrhosis.World J Gastroenterol. 2021 Jun 14;27(22):3050-3063. doi: 10.3748/wjg.v27.i22.3050. World J Gastroenterol. 2021. PMID: 34168407 Free PMC article. Review.
Cited by
-
Chinese Guidelines on the Management of Hepatic Encephalopathy in Cirrhosis (2024).J Clin Transl Hepatol. 2025 Mar 28;13(3):253-267. doi: 10.14218/JCTH.2024.00484. Epub 2025 Feb 17. J Clin Transl Hepatol. 2025. PMID: 40078200 Free PMC article.
-
Minimal encephalopathy in hereditary hemorrhagic telangiectasia patients with portosystemic vascular malformations.Orphanet J Rare Dis. 2024 Dec 21;19(1):484. doi: 10.1186/s13023-024-03493-3. Orphanet J Rare Dis. 2024. PMID: 39709450 Free PMC article.
-
The effects of rifaximin and lactulose on the gut-liver-brain axis in rats with minimal hepatic encephalopathy.PLoS One. 2025 Jun 17;20(6):e0325988. doi: 10.1371/journal.pone.0325988. eCollection 2025. PLoS One. 2025. PMID: 40526631 Free PMC article.
-
Sarcopenia in liver cirrhosis: perspectives from epigenetics and microbiota.Front Med (Lausanne). 2023 Oct 10;10:1264205. doi: 10.3389/fmed.2023.1264205. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37881635 Free PMC article. Review.
-
Research reviews and prospects of gut microbiota in liver cirrhosis: a bibliometric analysis (2001-2023).Front Microbiol. 2024 Mar 14;15:1342356. doi: 10.3389/fmicb.2024.1342356. eCollection 2024. Front Microbiol. 2024. PMID: 38550860 Free PMC article.
References
-
- Bajaj J. S., Fagan A., White M. B., Wade J. B., Hylemon P. B., Heuman D. M., et al. (2019). Specific gut and salivary microbiota patterns are linked with different cognitive testing strategies in minimal hepatic encephalopathy. Am. J. Gastroenterol. 114 1080–1090. 10.14309/ajg.0000000000000102 - DOI - PMC - PubMed
-
- Bajaj J. S., Heuman D. M., Hylemon P. B., Sanyal A. J., Puri P., Sterling R. K., et al. (2014a). Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment. Pharmacol. Ther. 39 1113–1125. 10.1111/apt.12695 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

