Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation
- PMID: 37033033
- PMCID: PMC10080024
- DOI: 10.3389/fpubh.2023.1098419
Looking back at prospective modeling of outbreak response strategies for managing global type 2 oral poliovirus vaccine (OPV2) cessation
Abstract
Introduction: Detection of poliovirus transmission and ongoing oral poliovirus vaccine (OPV) use continue to delay poliomyelitis eradication. In 2016, the Global Polio Eradication Initiative (GPEI) coordinated global cessation of type 2 OPV (OPV2) for preventive immunization and limited its use to emergency outbreak response. In 2019, GPEI partners requested restart of some Sabin OPV2 production and also accelerated the development of a genetically modified novel OPV2 vaccine (nOPV2) that promised greater genetic stability than monovalent Sabin OPV2 (mOPV2).
Methods: We reviewed integrated risk, economic, and global poliovirus transmission modeling performed before OPV2 cessation, which recommended multiple risk management strategies to increase the chances of successfully ending all transmission of type 2 live polioviruses. Following OPV2 cessation, strategies implemented by countries and the GPEI deviated from model recommended risk management strategies. Complementing other modeling that explores prospective outbreak response options for improving outcomes for the current polio endgame trajectory, in this study we roll back the clock to 2017 and explore counterfactual trajectories that the polio endgame could have followed if GPEI had: (1) managed risks differently after OPV2 cessation and/or (2) developed nOPV2 before and used it exclusively for outbreak response after OPV2 cessation.
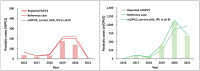
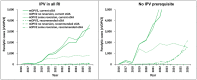
Results: The implementation of the 2016 model-based recommended outbreak response strategies could have ended (and could still substantially improve the probability of ending) type 2 poliovirus transmission. Outbreak response performance observed since 2016 would not have been expected to achieve OPV2 cessation with high confidence, even with the availability of nOPV2 prior to the 2016 OPV2 cessation.
Discussion: As implemented, the 2016 OPV2 cessation failed to stop type 2 transmission. While nOPV2 offers benefits of lower risk of seeding additional outbreaks, its reduced secondary spread relative to mOPV2 may imply relatively higher coverage needed for nOPV2 than mOPV2 to stop outbreaks.
Keywords: cessation; dynamic modeling; eradication; immunization; oral poliovirus vaccine; outbreak response; polio.
Copyright © 2023 Thompson, Kalkowska and Badizadegan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Updated Characterization of Outbreak Response Strategies for 2019-2029: Impacts of Using a Novel Type 2 Oral Poliovirus Vaccine Strain.Risk Anal. 2021 Feb;41(2):329-348. doi: 10.1111/risa.13622. Epub 2020 Nov 10. Risk Anal. 2021. PMID: 33174263 Free PMC article.
-
Serotype 2 oral poliovirus vaccine (OPV2) choices and the consequences of delaying outbreak response.Vaccine. 2023 Apr 6;41 Suppl 1(Suppl 1):A136-A141. doi: 10.1016/j.vaccine.2021.04.061. Epub 2021 May 14. Vaccine. 2023. PMID: 33994237 Free PMC article.
-
Updated Characterization of Post-OPV Cessation Risks: Lessons from 2019 Serotype 2 Outbreaks and Implications for the Probability of OPV Restart.Risk Anal. 2021 Feb;41(2):320-328. doi: 10.1111/risa.13555. Epub 2020 Jul 6. Risk Anal. 2021. PMID: 32632925 Free PMC article. Review.
-
Coordinated global cessation of oral poliovirus vaccine use: Options and potential consequences.Risk Anal. 2024 Feb;44(2):366-378. doi: 10.1111/risa.14158. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344934 Free PMC article. Review.
-
Outbreak response strategies with type 2-containing oral poliovirus vaccines.Vaccine. 2023 Apr 6;41 Suppl 1(Suppl 1):A142-A152. doi: 10.1016/j.vaccine.2022.10.060. Epub 2022 Nov 17. Vaccine. 2023. PMID: 36402659 Free PMC article.
Cited by
-
Increasing Population Immunity Prior to Globally-Coordinated Cessation of Bivalent Oral Poliovirus Vaccine (bOPV).Pathogens. 2024 Sep 17;13(9):804. doi: 10.3390/pathogens13090804. Pathogens. 2024. PMID: 39338995 Free PMC article.
-
Inactivated Poliovirus Vaccine: Recent Developments and the Tortuous Path to Global Acceptance.Pathogens. 2024 Mar 4;13(3):224. doi: 10.3390/pathogens13030224. Pathogens. 2024. PMID: 38535567 Free PMC article. Review.
-
Health Economic Analysis of Antiviral Drugs in the Global Polio Eradication Endgame.Med Decis Making. 2023 Oct-Nov;43(7-8):850-862. doi: 10.1177/0272989X231191127. Epub 2023 Aug 14. Med Decis Making. 2023. PMID: 37577803 Free PMC article.
-
Worst-case scenarios: Modeling uncontrolled type 2 polio transmission.Risk Anal. 2024 Feb;44(2):379-389. doi: 10.1111/risa.14159. Epub 2023 Jun 21. Risk Anal. 2024. PMID: 37344376 Free PMC article.
-
Review of Poliovirus Transmission and Economic Modeling to Support Global Polio Eradication: 2020-2024.Pathogens. 2024 May 22;13(6):435. doi: 10.3390/pathogens13060435. Pathogens. 2024. PMID: 38921733 Free PMC article.
References
-
- Thompson KM, Segui-Gomez M, Graham JD. Validating analytical judgments: the case of the airbag's lifesaving effectiveness. Reliability Eng Syst Safety. (1999) 66:57–68. 10.1016/S0951-8320(99)00019-8 - DOI
-
- World Health Organization. Global Polio Eradication Initiative. Circulating Vaccine-Derived Poliovirus. (2023). Available online at: https://polioeradication.org/wp-content/uploads/2023/02/weekly-polio-ana... (accesssed February 24, 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

