A simplified nasopharyngeal swab collection procedure for minimizing patient discomfort while retaining sample quality
- PMID: 37033037
- PMCID: PMC10076767
- DOI: 10.3389/fpubh.2023.1066934
A simplified nasopharyngeal swab collection procedure for minimizing patient discomfort while retaining sample quality
Abstract
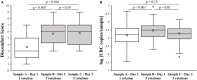
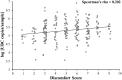
A nasopharyngeal swab (NPS) is the most frequently collected sample type when molecular diagnosis of respiratory viruses, including SARS CoV-2, is required. An optimal collection technique would provide sufficient sample quality for the diagnostic process and would minimize the discomfort felt by the patient. This study compares a simplified NPS collection procedure with only one rotation of the swab to a more standard procedure with five rotations. Swabs were collected from 76 healthy volunteers by the same healthcare professional on 2 consecutive days at a similar hour to minimize variability. The number of Ubiquitin C copy number per sample was measured by real-time quantitative PCR and patient discomfort was assessed by questionnaire. No statistically significant difference (p = 0.15) was observed in the Ubiquitin C copy number per sample between a NPS collected with one rotation (5.2 ± 0.6 log UBC number copies/sample) or five rotations (5.3 ± 0.5 log UBC number copies/sample). However, a statistically significant difference was observed in discomfort between these two procedures, the second being much more uncomfortable. Additional analysis of the results showed a weak correlation between discomfort and the number of human cells recovered (Spearman's rho = 0.202) and greater discomfort in younger people. The results of this study show that a NPS collected with one slow rotation has the same quality as a NPS collected with five rotations. However, the collection time is shorter and, most importantly, less unpleasant for patients.
Keywords: Ubiquitin C; collection procedure; discomfort score; nasopharyngeal swab; sample quality.
Copyright © 2023 Uršič, Kogoj, Šikonja, Jevšnik Virant and Petrovec.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Organization WH . Interim Guidance 19 March 2020. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases. Geneva:WHO (2020).
-
- Center for Disease Control Prevention. Interim Guidlines for Collecting and Handling of Clinical Specimens for COVID-19 testing. (2022). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

