A new era for optic pathway glioma: A developmental brain tumor with life-long health consequences
- PMID: 37033188
- PMCID: PMC10080591
- DOI: 10.3389/fped.2023.1038937
A new era for optic pathway glioma: A developmental brain tumor with life-long health consequences
Abstract
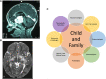
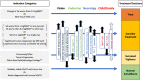
Optic pathway and hypothalamic glioma (OPHG) are low-grade brain tumors that arise from any part of the visual pathways frequently involving the hypothalamus. The tumors grow slowly and present with features driven by their precise anatomical site, their age at presentation and the stage of growth and development of the host neural and orbital bony tissues. Up to 50% of optic pathway glioma arise in association with Neurofibromatosis type 1 (NF1), which affects 1 in 3,000 births and is a cancer predisposition syndrome. As low-grade tumors, they almost never transform to malignant glioma yet they can threaten life when they present under two years of age. The main risks are to threaten vision loss by progressive tumor damage to optic pathways; furthermore, invasion of the hypothalamus can lead to diencephalic syndrome in infancy and hypopituitarism later in life. Progressive cognitive and behavioural dysfunction can occur, as part of NF1 syndromic features and in sporadic cases where large bulky tumors compress adjacent structures and disrupt neuro-hypothalamic pathways. Persistently progressive tumors require repeated treatments to attempt to control vision loss, other focal brain injury or endocrine dysfunction. In contrast tumors presenting later in childhood can be seen to spontaneously arrest in growth and subsequently progress after periods of stability. These patterns are influenced by NF status as well as stages of growth and development of host tissues. The past two decades has seen an expansion in our understanding and knowledge of the clinical and scientific features of these tumors, their modes of presentation, the need for careful visual and endocrine assessment. This influences the decision-making surrounding clinical management with surgery, radiotherapy, chemotherapy and most recently, the potential benefit of molecularly targeted drug therapy. This article, based upon the authors' clinical and research experience and the published literature will highlight advances in approach to diagnosis, the established role of vision loss as justification of treatments and the emerging evidence of endocrine and neurological consequences that need to be incorporated into judgements for case selection for therapy or observation. Consideration is given to the current state of biological evidence justifying current trials of new therapies, the genetic studies of the NF1 gene and the potential for new approaches to OPHG detection and treatment. The outstanding health system priorities from the perspective of children, their parents and health system commissioners or insurers are discussed.
Keywords: childhood; endocrine late effects; health outcomes; optic pathway hypothalamic glioma; treatment selection; vision loss.
© 2023 Walker, Aquilina, Spoudeas, Pilotto, Gan and Meijer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Walker DA, Liu J, Kieran M, Jabado N, Picton S, Packer R, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the delphi method. Neuro Oncol. (2013) 15(4):462–8. 10.1093/neuonc/nos330 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

